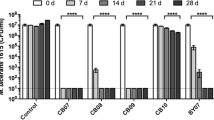
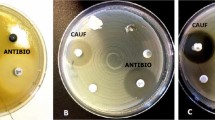
Abstract
Antibacterial clays in nature include a variety of clay mineral assemblages that are capable of killing certain human pathogens. Although clays have been used for medicinal applications historically, only in the last decade have analytical methods and instrumentation been developed that allow researchers to evaluate the antibacterial mechanisms of various clays applied medicinally. Comparisons of the mineralogical and chemical compositions of natural clays that kill bacteria have promoted a better understanding of the mineral properties that are toxic to a broad-spectrum of human pathogens, including bacteria that have developed resistance to antibiotics. Popular literature is filled with reports of ‘healing’ clays, that, when tested against pathogens in vitro and compared to controls, do not appear to have bactericidal properties. It is important, however, to differentiate what properties make a clay ‘healing,’ versus what makes a clay ‘antibacterial.’ Most antibacterial clays identified to date buffer pH conditions of a hydrated clay outside the range of conditions in which human pathogens thrive (circum-neutral pH) and require oxidation reactions to occur. It is the change in oxidation state and pH imposed by the hydrated clay, applied topically, that leads to a chemical attack of the bacteria. Healing clays, on the other hand, may not kill bacteria but have soothing effects that are palliative. This article reviews some of the historical uses of clays in medicine but focuses primarily on the common characteristics of natural antibacterial clays and early studies of their antibacterial mechanisms. In this era of bacterial resistance to antibiotics, mimicking the antibacterial mechanisms exhibited by natural clays could be advantageous in the development of new antimicrobial agents.




















Similar content being viewed by others
REFERENCES
Alba, B. M., & Gross, C. A. (2004). Regulation of the Escherichia coli sigma-dependent envelope stress response. Molecular Microbiology, 52, 613–619.
Awad, M. E., López-Galindo, A., Setti, M., El-Rahmany, M. M., & Iborra, C. V. (2017). Kaolinite in pharmaceutics and biomedicine. International Journal of Pharmacology, 533, 34–48.
Baker, S. E., Sawvel, A. M., Zheng, N., & Stucky, G. D. (2007). Controlling bioprocesses with inorganic surfaces: Layered clay hemostatic agents. Chemistry of Materials, 19, 4390–4392.
Bauer, A. W., Kirby, W. M. M., Sherris, J. C., & Turck, M. (1966). Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology, 45, 493–496.
Behroozian, S., Svensson, S. L., & Davies, J. (2016). Kisameet clay exhibits potent antibacterial activity against the ESKAPE pathogens. mBio, 7, e01842-01815.
Caflisch, K. M., Schmidt-Malan, S. M., Mandrekar, J. N., Karau, M. J., Kicklas, J. P., Williams, L. B., & Patel, R. (2018). Antimicrobial activity of reduced iron clay against pathogenic biofilms from wound infections. International Journal of Antimicrobial Agents, 52, 692–696.
Carretero, M. I. (2002). Clay minerals and their beneficial effects upon human health: a review. Applied Clay Science, 21, 155–163.
Carretero, M. I., Gomes, C. S. F., & Tateo, F. (2006). Clays and human health. In F. Bergaya, B. K. G. Theng, & G. Lagaly (Eds.), Handbook of Clay Science (Vol. 1, pp. 717–741). Developments in Clay Science, Elsevier Ltd..
Cervini-Silva, J., Nieto-Camacho, A., Palacios, E., Montoya, J. A., Gómez-Vidales, V., & Ramirez-Apán, M. T. (2013). Anti-inflammatory and anti-bacterial activity, and cytotoxicity of halloysite surfaces. Colloids and Surfaces B, 111, 651–655.
Cervini-Silva, J., Nieto-Camacho, A., Kaufhold, S., Ufer, K., Palacios, E., Montoya, A., & Dathe, W. (2016). Antiphlogistic effect by zeolite as determined by a murine inflammation model. Microporous and Mesoporous Materials, 228, 207–214.
CLSI, [formerly National Committee for Clinical Laboratory Standards: NCCLS] (1999). Methods for Determining Bactericidal Activity of Antimicrobial Agents: Approved Guideline. NCCLS document M26-A, 19(18), 32pp.
CLSI, [Clinical and Laboratory Standards Institute] (2012). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: Approved Standard, 9th edition. M07-A9, 32(2), 69pp.
Cunningham, T. M., Koehl, J. L., Summers, J. S., & Haydel, S. E. (2010). pH-dependent metal ion toxicity influences the antibacterial activity of two natural mineral mixtures. Public Library of Science One, 5, e9456.
Cygan R. T., Ho C. K., & Weiss C. J. (2002). Linking the Geosciences to Emerging Bio-engineering Technologies. Sandia National Laboratories Report SAND2002–3690, 59 pp.
Dong, H. L., Fredrickson, J. K., Kennedy, D. W., Zachara, J. M., Kukkadappu, R. K., & Onstott, T. C. (2000). Mineral transformation associated with the microbial reduction of magnetite. Chemical Geology, 169, 299–318.
Eberl, D. D. (2003). Users guide to RockJock: A program for determining quantitative mineralogy from powder X-ray diffraction data. USGS Open file Report 03-78. USGS.
Ernstsen, V., Gates, W. P., & Stucki, J. W. (1998). Microbial reduction of structural iron in clays – A renewable source of reduction capacity. Journal of Environmental Quality, 27, 761–766.
Exley, C. (2004). The pro-oxidant activity of aluminum. Free Radical Biology in Medicine, 36, 380–387.
Fein, J., Daughney, C., Yee, N., & Davis, T. A. (1997). A chemical equilibrium model for metal adsorption onto bacterial surfaces. Geochimica et Cosmochimica Acta, 67, 3319–3328.
Fenton, H. J. H. (1894). Oxidation of tartaric acid in the presence or iron. Journal of the Chemical Society, 65, 899–910.
Ferrell, R. E., Jr. (2008). Medicinal clay and spiritual healing. Clays and Clay Minerals, 56, 751–760.
Ferris, F. G., Fyfe, W. S., & Beveridge, T. J. (1987). Bacteria as nucleation sites for authigenic minerals in a metal-contaminated lake sediment. Chemical Geology, 63, 225–232.
Finkelman, R. B. (2006). Health benefits of geologic materials and geologic processes. International Journal of Environmental Research and Public Health, 3, 338–342.
Finkelman, R. B. (2019). The influence of clays on human health: A medical geology perspective. Clays and Clay Minerals, 67.
Fortin, D., & Beveridge, T. J. (1997). Role of the bacterium Thiobacillus in the formation of silicates in acid mine tailings. Chemical Geology, 141, 235–250.
George, K. M., Chatterjee, D., Gunawardana, G., Welty, D., Hayman, J., Lee, R., & Small, P. L. C. (2002). Mycolactone: A polyketide toxin from Mycobacterium ulcerans, required for virulence. Science, 283(5403), 854–857.
Gomes, C. (2013). Naturotherapies based on minerals. Geomaterials, 3, 1–14.
Haack, E. A., & Warren, L. A. (2003). Biofilm hydrous manganese oxyhydroxides and metal dynamics in acid rock drainage. Environmental Science & Technology, 37, 4138–4147.
Harrison, J. J., Turner, R. J., & Ceri, H. (2005). High-throughput metal susceptibility testing of microbial biofilms. BMC Microbiology, 5, 1–11.
Haydel, S. E., Remenih, C. M., & Williams, L. B. (2008). Broad-spectrum in vitro antibacterial activities of clay minerals against antibiotic-susceptible and antibiotic-resistant bacterial pathogens. Journal of Antimicrobial Chemotherapy, 61, 353–361.
Hedges, A. J. (2002). Estimating the precision of serial dilutions and viable bacterial counts. International Journal of Food Microbiology, 76, 207–214.
Høiby, N., Bjarnsholt, T., Givskov, M., Molin, S., & Coifu, O. (2010). Antibiotic resistance of bacterial biofilms. International Journal of Antimicrobial Agents, 35, 322–332.
Hoorn, C. (1994). Fluvial palaeoenvironments in the intracratonic Amazonas Basin (Early Miocene-early Middle Miocene, Colombia). Palaeogeography, Palaeoclimatology, Palaeoecology, 109, 1–54.
Honty, M., De Craen, M., Wang, L., Madejova, J., Czimerova, A., Pentrak, M., Stricek, I., & Van Geet, M. (2010). The effect of high pH alkaline solutions on the mineral stability of the Boom Clay – Batch experiments at 60°C. Applied Geochemistry, 25, 825–840.
Huisman, O., D'Ari, R., & Gottesman, S. (1984). Cell-division control in Escherichia coli: Specific induction of the SOS function SfiA protein is sufficient to block septation. Proceedings of the National Academy of Science USA, 81, 4490–4494.
Imlay, J. A., Chin, S. M., & Linn, S. (1988). Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science, 240, 640–642.
Jackson, M. L. (1979). Soil Chemical Analysis Advanced course. 2nd edition. Madison : Published by the author. 895 pp.
Keyer, K., & Imlay, J. A. (1996). Superoxide accelerates DNA damage by elevating free-iron levels. Proceedings of the National Academy of Science USA, 93, 13635–13640.
Kibanova, D., Nieto-Camacho, A., & Cervini-Silva, J. (2009). Lipid peroxidation induced by expandable clay minerals. Environmental Science & Technology, 43, 7550–7555.
Kim, J., Dong, H., Seabaugh, J., Newell, S. W., & Eberl, D. D. (2004). Role of microbes in the smectite-to-illite reaction. Science, 203, 830–832.
Kogel, J. E. (2014). Mining and processing kaolin. Elements, 10, 189–193.
Kohanski, M. A., Dwyer, D. J., Hayete, B., Lawrence, C. A., & Collins, J. J. (2007). A common mechanism of cellular death induced by bactericidal antibiotics. Cell, 130, 797–810.
Konhauser, K. O. (2007). Introduction to Geomicrobiology (425 p). Oxford: Blackwell Publishing.
Konhauser, K. O., & Urrutia, M. M. (1999). Bacterial clay authigenesis: a common biogeochemical process. Chemical Geology, 161, 399–413.
Kostka, J. E., Dalton, D. D., Skelton, H., Dollhopf, S., & Stucki, J. W. (2002). Growth of iron (III)-reducing bacteria on clay minerals as the sole electron acceptor and comparison of growth yields on a variety of oxidized iron forms. Applied Environmental Microbiology, 68, 6256–6262.
Laufer, B. (1930). Geophagy. Field Museum of Natural History, Chicago, USA, Publication 280, 198 pp.
Lawton, G., Granville-Chapman, J., & Parker, P. J. (2009). Novel haemostatic dressings. Journal of the Royal Army Medical Corps, 155, 309–314.
Lemire, J. A., Harrison, J. J., & Turner, R. J. (2013). Antimicrobial activity of metals: mechanisms, molecular targets and applications. National Review of Microbiology, 11, 371–384.
Londoño, S. C., & Williams, L. B. (2016). Unraveling the antibacterial mode of action of a clay from the Colombian Amazon. Environmental Geochemistry and Health, 38, 363–379.
Londoño, S. C., Hartnett, H. E., & Williams, L. B. (2017). Antibacterial activity of aluminum in clay from the Colombian Amazon. Environmental Science & Technology, 51, 2401–2408.
Ma’or, Z., Henis, Y., Alon, Y., Orlov, E., Sørensen, K. B., & Oren, A. (2006). Antimicrobial properties of Dead Sea black mineral mud. International Journal of Dermatology, 45, 504–511.
Maurice, P. A., & Warren, L. A. (2006). Introduction to geomicrobiology: microbial interactions with minerals. In P. A. Maurice & L. A. Warren (Eds.), Methods for Study of Microbe-mineral Interactions (Vol. 14, pp. 2–35). Clay Minerals Society Workshop Lectures.
Moore, D. M., & Reynolds, R. C. (1997). X-ray Diffraction and the Identification and Analysis of Clay Minerals (2nd ed.378 pp). New York: Oxford University Press.
Morrison, K. D., Underwood, J. C., Metge, D. W., Eberl, D. D., & Williams, L. B. (2014). Mineralogical variables that control the antibacterial effectiveness of a natural clay deposit. Environmental Geochemistry and Health, 36, 613–631.
Morrison, K. D., Misra, R., & Williams, L. B. (2016). Unearthing the antibacterial mechanism of medicinal clay: A geochemical approach to combating antibiotic resistance. Nature Scientific Reports, 6, 19043.
Morrison, K. D., Williams, S. N., & Williams, L. B. (2017). The anatomy of an antibacterial clay deposit: A new economic geology. Economic Geology, 112, 1551–1570.
Nealson, K. H., Belz, A., & McKee, B. (2002). Breathing metals as a way of life: geobiology in action. Antonie Van Leeuwenhoek International Journal of General and Molecular Microbiology, 81, 215–222.
Nies, D. H. (1999). Microbial heavy-metal resistance. Applied Microbiology and Biotechnology, 51, 730–750.
Nunez, J., Renslow, R., Cliff, J. B. III, & Anderton, C. R. (2018). NanoSIMS for biological applications: Current practices and analyses. Biointerphases 03B301, 13, 1–26.
Pearson, R. G. (1966). Acids and bases. Science, 151, 172–177.
Potts, R., Behrensmeyer, A. K., Faith, J. T., Tryon, C. A., Brooks, A. S., Yellen, J. E., Deino, A. L., Kinyanjui, R., Clark, J. B., Haradon, C., Levin, N. E., Jeijer, H. H. M., Veatch, E. G., Owen, R. B., & Renaut, R. W. (2018). Environmental dynamics during the onset of the Middle Stone Age in eastern Africa. Science. https://doi.org/10.1126/science.aao2200.
Radomski, A., Jurasz, P., Alonso-Escolano, D., Drews, M., Morandi, M., Malinski, T., & Radomski, M. W. (2005). Nanoparticle-induced platelet aggregation and vascular thrombosis. British Journal of Pharmacology, 146, 882–893.
Raivio, T. L. (2005). Envelope stress responses and Gram-negative bacterial pathogenesis. Molecular Microbiology, 56, 1119–1128.
Reinbacher, W. R. (2003). Healing Earths; The Third Leg of Medicine (244 pp). Toronto, Canada: 1st Books Library, York University.
Schoonen, M. A. A., Cohn, C. A., Roemer, E., Laffers, R., Simon, S. R., & O’Riordan, T. (2006). Mineral-induced formation of reactive oxygen species. In N. Sahai & M. A. A. Schoonen (Eds.), Medical Mineralogy and Geochemistry (pp. 179–221). Chantilly: Reviews in Mineralogy and Geochemistry, 64, Mineralogical Society of America and Geochemical Society.
Schoonen, M. A. A., Harrington, A. D., Laffers, R. A., & Strongin, D. R. (2010). Role of hydrogen peroxide and hydroxyl radical in pyrite oxidation by molecular oxygen. Geochimica et Cosmochimica Acta, 74, 4971–4987.
Sezonov, G., Joseleau-Petit, D., & D’Ari, R. (2007). Escherichia coli physiology in Luria-Bertani broth. Journal of Bacteriology, 189, 8746–8749.
Sposito, G. (1980). The operational definition of the zero point of charge in soils. Soil Science Society of America Journal, 45, 292–297.
Środoń, J., & McCarty, D. K. (2008). Surface area and layer charge of smectite from CEC and EGME/H2O-retention measurements. Clays and Clay Minerals, 56, 155–174.
Stephens, C. (2002). Microbiology: Breaking down biofilms. Current Biology, 12, R132–R134.
Svensson, S. L., Behroozian, S., Xu, W., Surette, M. G., Li, L., & Davies, J. (2017). Kisameet glacial clay: an unexpected source of bacterial diversity. American Society of Microbiology mBio, 8, e00590–e00517.
Takeno, N. (2005). Atlas of Eh-pH diagrams: Intercomparison of thermodynamic databases. Geological Survey of Japan Open File Report No., 419, 285 pp.
Tateo, F., Ravaglioli, A., Andreoli, C., Bonina, F., Coiro, V., Degetto, S., Giaretta, A., Orsini, A. M., Puglia, C., & Summa, V. (2009). The in-vitro percutaneous migration of chemical elements from a thermal mud for healing use. Applied Clay Science, 44, 83–94.
Valko, M., Morris, H., & Cronin, M. T. D. (2005). Metals, toxicity and oxidative stress. Current Medicinal Chemistry, 12, 1161–1208.
Vermeer, D. E., & Ferrell, R. E., Jr. (1985). Nigerian geophagical clay: a traditional antidiarrheal pharmaceutical. Science, 227, 634–636.
Walker, S., Flemming, C., Ferris, F., Beveridge, T., & Bailey, G. (1989). Physicochemical interaction of Escherichia coli cell envelopes and Bacillus subtilis cell walls with two clays and ability of the composite to immobilize heavy metals from solution. Applied and Environmental Microbiology, 55, 2976–2984.
Wang, X., Dong, H., Zeng, Q., Xia, Q., Zhang, L., & Zhou, Z. (2017). Reduced iron-containing clay minerals as antibacterial agents. Environmental Science & Technology, 51, 7639–7647.
Williams, R. J. (1999). What is wrong with aluminum? Journal of Inorganic Biochemistry, 76, 81–88.
Williams, L. B. (2017). Geomimicry: Harnessing the antibacterial action of clays. Clay Minerals, 52, 1–24.
Williams, L. B., & Haydel, S. E. (2010). Evaluation of the medicinal use of clay minerals as antibacterial agents. International Geology Reviews, 52, 745–770.
Williams, L. B., & Hillier, S. (2014). Kaolins and Health: from first grade to first aid. Elements, 10, 207–211.
Williams, L. B., Holland, M., Eberl, D. D., Brunet, T., & Brunet de Courrsou, L. B. (2004). Killer clays! Natural antibacterial clay minerals. Mineralogical Society Bulletin, 139, 3–8.
Williams, L. B., Haydel, S. E., Giese, R. F., & Eberl, D. D. (2008). Chemical and mineralogical characteristics of French green clays used for healing. Clays and Clay Minerals, 56, 437–452.
Williams, L. B., Metge, D. W., Eberl, D. D., Harvey, R. W., Turner, A. G., Prapaipong, P., & Poret-Peterson, A. T. (2011). What makes a natural clay antibacterial? Environmental Science & Technology, 45, 3768–3773.
Wilson, M. J. (2003). Clay mineralogical and related characteristics of geophagic materials. Journal of Chemical Ecology, 29, 1525–1547.
Wilson, E., Henry, D. A., & Smith, J. A. (1990). Disk elution method for MICs and MBCs. Antimicrobial Agents and Chemotherapy, 34, 2128–2132.
Winterbourn, C. C. (1995). Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicology Letters, 82−83, 969–974.
Winterbourn, C. C. (2008). Reconciling the chemistry and biology of reactive oxygen species. Nature Chemical Biology, 4, 278–286.
Young, S. L. (2011). Craving Earth (228 pp). New York: Columbia University Press.
Zarate-Reyes, L., Nieto-Camacho, A., Palacios, E., Gomaz-Vidales, V., Kaufhold, S., Ufer, K., Garcia-Zepeda, E., & Cervini-Silva, J. (2017). Antibacterial clay against Gram-negative antibiotic resistant bacteria. Journal of Hazardous Materials, 342, 625–632.
Zastawny, T. H., Altman, S. A., Randerseichhorn, L., Madurawe, R., Lumpkin, J. A., Dizdaroglu, M., & Rao, G. (1995). DNA base modifications and membrane damage in cultured mammalian cells treated with iron ions. Free Radical Biology in Medicine, 18, 1013–1022.
ACKNOWLEDGMENTS
This research has been supported by the US National Institutes of Health (NIH R21 AT003618), National Science Foundation research grants (NSF EAR-1123931 and NSF EAR-1719325), and the ASU SIMS Facility, which is supported by NSF grant EAR 1352996. Many colleagues and students contributed to the results presented in this review and their contributions are greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
(Received 16 April 2018; revised 3 December 2018; AE: Jin-Ho Choy)
Rights and permissions
About this article
Cite this article
Williams, L.B. NATURAL ANTIBACTERIAL CLAYS: HISTORICAL USES AND MODERN ADVANCES. Clays Clay Miner. 67, 7–24 (2019). https://doi.org/10.1007/s42860-018-0002-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42860-018-0002-8