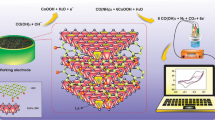
Abstract
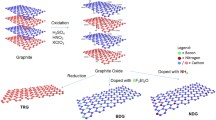
A simple and one-pot synthetic procedure using two different sources has been demonstrated to prepare heteroatoms doped reduced graphene oxide such as nitrogen-doped reduced graphene oxide (N-RGO) and sulfur-doped reduced graphene oxide (S-RGO). The N-RGO has been hydrothermally synthesized using urea as nitrogen precursor, wherein the S-RGO has been synthesized using dimethyl sulfoxide (DMSO) as sulfur precursor. The successful N-doping, S-doping and other physicochemical properties of N-RGO and S-RGO have been confirmed with different spectroscopic and electrochemical techniques. The results indicated that doping into the graphene structure exhibits a high conductivity and a better transfer of charge. Moreover, heteroatoms doped graphene (N-RGO and S-RGO) and graphene-related materials (RGO) have been applied for the individual detection of uric acid (UA). Interestingly, the N-RGO exhibited a lower limit of detection (LOD, S/N = 3) of 2.7 10–5 M for UA (10–1000 µM) compared with undoped RGO and S-RGO. Furthermore, the simultaneous detection of UA in the presence of Xanthine (XA) has been demonstrated a wide linear range of detection for UA: 10–1000 µM, with unchanged concentration of XA to be 200 µM, and exhibited a low limit of detection of 8.7 10−5 M (\(S/N\) = 3) for UA. This modified sensor based on N-RGO has revealed a high selectivity and reproducibility thanks to its large surface area, high catalytic properties, and chemical structure. Indeed, the practical applicability of the proposed sensor has been evaluated in milk samples even in the presence of high concentrations of UA with satisfactory results.
Graphical Abstract
















Similar content being viewed by others
Data availability
Data will be sent by the authors if requested.
References
Hafez RM, Abdel-Rahman TM, Naguib RM (2017) Uric acid in plants and microorganisms: biological applications and genetics—a review. J Adv Res 8(5):475–486. https://doi.org/10.1016/j.jare.2017.05.003
Curulli A (2021) Electrochemical biosensors in food safety: challenges and perspectives. Molecules 26(10):2940. https://doi.org/10.3390/molecules26102940
Motshakeri M, Phillips ARJ, Travas-Sejdic J, Kilmartin PA (2020) Electrochemical study of gold microelectrodes modified with PEDOT to quantify uric acid in milk samples. Electroanalysis. https://doi.org/10.1002/elan.202060086
Moteshakeri M, Sejdic JT, Kilmartin PA (2018) Effect of holding time on electrochemical analysis of milk antioxidants using PEDOT electrodes. Int J Nanotechnol 15(8/9/10):729. https://doi.org/10.1504/ijnt.2018.098441
Larsen T, Moyes KM (2010) Fluorometric determination of uric acid in bovine milk. J Dairy Res 77(04):438–444. https://doi.org/10.1017/s0022029910000580
Huang S-H, Liao H-H, Chen D-H (2010) Simultaneous determination of norepinephrine, uric acid, and ascorbic acid at a screen printed carbon electrode modified with polyacrylic acid-coated multi-wall carbon nanotubes. Biosens Bioelectron 25(10):2351–2355. https://doi.org/10.1016/j.bios.2010.03.028
Wang X, Chen S, Tang X, Lin D, Qiu P (2019) Ultrasensitive detection of uric acid in serum of patients with gout by a new assay based on Pt@Agnanoflowers. RSC Adv 9(63):36578–36585. https://doi.org/10.1039/c9ra06481h
Tana C, Ticinesi A, Prati B, Nouvenne A, Meschi T (2018) Uric acid and cognitive function in older individuals. Nutrients 10(8):975. https://doi.org/10.3390/nu10080975
Wu S, Jia S, Dong X (2016) Study on detection methods for xanthine in food and biological samples. Int J Curr Res Chem Pharm Sci 3(8):1–5
Bjerre-Harpøth V, Friggens NC, Thorup V, Larsen T, Moyes M (2012) Metabolic and production profiles of dairy cows in response to decreased nutrient density to increase physiological imbalance at different stages of lactation. J Dairy Sci 95(5):2362–2380. https://doi.org/10.3168/jds.2011-4419
Demirkan B, Bozkurt S, Şavk A, Cellat K, Gülbağca F, Nas MS et al (2019) Composites of bimetallic platinum-cobalt alloy nanoparticles and reduced graphene oxide for electrochemical determination of ascorbic acid, dopamine, and uric acid. Sci Rep. https://doi.org/10.1038/s41598-019-48802-0
Lian D-S, Zhao S-J (2016) Capillary electrophoresis based on nucleic acid detection for diagnosing human infectious disease. Clin Chem Lab Med (CCLM). https://doi.org/10.1515/cclm-2015-0096
Kanďár R, Drábková P, Hampl R (2011) The determination of ascorbic acid and uric acid in human seminal plasma using an HPLC with UV detection. J Chromatogr B 879(26):2834–2839. https://doi.org/10.1016/j.jchromb.2011.08.007
Adriano S, Jason JD, Bueno PR (2014) Fundamentals and applications of impedimetric and redox capacitive biosensors. J Anal Bioanal Techn. https://doi.org/10.4172/2155-9872.S7-016
Sunil K, Abhay N (2021) Application of carbon nanomaterials decorated electrochemical sensor for analysis of environmental pollutants. Anal Chem Adv Perspect Appl. https://doi.org/10.5772/intechopen.96538
Attoye B, Baker MJ, Thomson F, Pou C, Corrigan DK (2021) Optimisation of an electrochemical DNA sensor for measuring KRAS G12D and G13D point mutations in different tumour types. Biosensors 11(2):42. https://doi.org/10.3390/bios11020042
Omar WIW, Soon CF, Ahmad MK, Shimomura M (2021) Hydrothermal synthesis of biocompatible nitrogen doped graphene quantum dots. Energy Environ. https://doi.org/10.1177/0958305x20984112
Vinodhkumar G, Ramya R, Potheher I, Peter C (2018) Reduced graphene oxide based on simultaneous detection of neurotransmitters. Prog Chem Biochem Res 1:40–49. https://doi.org/10.29088/SAMI/PCBR.2018.1.4049
Yi S-Y, Lee J-H, Hong H-G (2013) A selective determination of levodopa in the presence of ascorbic acid and uric acid using a glassy carbon electrode modified with reduced graphene oxide. J Appl Electrochem 44(5):589–597. https://doi.org/10.1007/s10800-013-0649-8
Wang S, Zhang L, Xia Z, Roy A, Chang DW, Baek J-B, Dai L (2012) BCN graphene as efficient metal-free electrocatalyst for the oxygen reduction reaction. Angew Chemie Int Ed 51(17):4209–4212. https://doi.org/10.1002/anie.201109257
Chen R, Wang Y, Liu Y, Li J (2015) Selective electrochemical detection of dopamine using nitrogen-doped graphene/manganese monoxide composites. RSC Adv 5(103):85065–85072. https://doi.org/10.1039/c5ra14328d
Zhai C, Sun M, Zhu M, Song S, Jiang S (2017) A new method to synthesize sulfur-doped graphene as effective metal-free electrocatalyst for oxygen reduction reaction. Appl Surf Sci 407:503–508. https://doi.org/10.1016/j.apsusc.2017.02.191
Vinodha S, Vidhya P, Ramya T (2020) Graphene metal modified electrochemical sensors for toxic chemicals. Mater Res Found. https://doi.org/10.21741/9781644900956-4
Wang Y, Hu M, Ai D, Zhang H, Huang Z-H, Lv R, Kang F (2019) Sulfur-doped reduced graphene oxide for enhanced sodium ion pseudocapacitance. Nanomaterials 9(5):752. https://doi.org/10.3390/nano9050752
Zhao Z, Mei T, Chen Y, Qiu J, Xu D, Wang J et al (2015) One-pot synthesis of lightweight nitrogen-doped graphene hydrogels with supercapacitive properties. Mater Res Bull 68:245–253. https://doi.org/10.1016/j.materresbull.2015.03.062
Hsine Z, Bizid S, Zahou I, Ben Haj Hassen L, Nasri H, Mlika R (2018) A highly sensitive impedimetric sensor based on iron (III) porphyrin and thermally reduced graphene oxide for detection of Bisphenol A. Synth Met 244:27–35. https://doi.org/10.1016/j.synthmet.2018.06.01
García-Miranda Ferrari A, Foster C, Kelly P, Brownson D, Banks C (2018) Determination of the electrochemical area of screen-printed electrochemical sensing platforms. Biosensors 8(2):53. https://doi.org/10.3390/bios8020053
Ambrosi A, Chua CK, Latiff NM, Loo AH, Wong CHA, Eng AYS, Pumera M (2016) Graphene and its electrochemistry—an update. Chem Soc Rev 45(9):2458–2493. https://doi.org/10.1039/c6cs00136j
Çiplak Z, Yildiz N, Çalimli A (2014) Investigation of graphene/Ag nanocomposites synthesis parameters for two different synthesis methods. Fullerenes, Nanotubes, Carbon Nanostruct 23(4):361–370. https://doi.org/10.1080/1536383X.2014.894025
Karikalan N, Karthik R, Chen S-M, Karuppiah C, Elangovan A (2017) Sonochemical synthesis of sulfur doped reduced graphene oxide supported CuS nanoparticles for the non-enzymatic glucose sensor applications. Sci Rep. https://doi.org/10.1038/s41598-017-02479-5
Gliniak J, Lin J-H, Chen Y-T, Li C-R, Jokar E, Chang C-H et al (2017) Sulfur-doped graphene oxide quantum dots as photocatalysts for hydrogen generation in the aqueous phase. Chemsuschem 10(16):3260–3267. https://doi.org/10.1002/cssc.201700910
Wu K, Feng Y, Li Y, Li L, Liu R, Zhu L (2020) S-doped reduced graphene oxide: a novel peroxidase mimetic and its application in sensitive detection of hydrogen peroxide and glucose. Anal Bioanal Chem. https://doi.org/10.1007/s00216-020-02767-6
Richa S, Kumar P, Mahesh S, Kumar R, Satinder S (2020) Highly sensitive electrochemical sensing of neurotransmitter dopamine from scalable UV irradiation-based nitrogen-doped reduced graphene oxide-modified electrode. Bull Mater Sci. https://doi.org/10.1007/s12034-020-02091-w
Rochman RA, Wahyuningsih S, Ramelan AH, Hanif QA (2019) Preparation of nitrogen and sulphur Co-doped reduced graphene oxide (rGO-NS) using N and S heteroatom of thiourea. IOP Conf Ser: Mater Sci Eng 509:012119. https://doi.org/10.1088/1757-899x/509/1/012119
Porwal J, Karanwal N, Kaul S, Jain SL (2016) Carbocatalysis: N-doped reduced graphene oxide catalyzed esterification of fatty acids with long chain alcohols. N J Chem 40(2):1547–1553. https://doi.org/10.1039/c5nj02095f
Sun S-W, Liu H-L, Zhou Y, Wang F-B, Xia X-H (2017) Copper–nitrogen-doped graphene hybrid as an electrochemical sensing platform for distinguishing DNA bases. Anal Chem 89(20):10858–10865. https://doi.org/10.1021/acs.analchem.7b02520
Shanmugasundaram A, Gundimeda V, Hou T, Lee DW (2017) Realizing synergy between In2O3 nanocubes and nitrogen-doped reduced graphene oxide: an excellent nanocomposite for the selective and sensitive detection of CO at ambient temperatures. ACS Appl Mater Interfaces 9(37):31728–31740. https://doi.org/10.1021/acsami.7b06253
Shu R, Wan Z, Zhang J, Wu Y, Liu Y, Shi J-J, Zheng M (2019) Facile design of three-dimensional nitrogen-doped reduced graphene oxide/multi-walled carbon nanotubes composite foams as lightweight and high-efficient microwave absorbers. ACS Appl Mater Interfaces. https://doi.org/10.1021/acsami.9b16134
Yang Z-Z, Zheng Q-b, Qiu H-x, Yang J (2015) A simple method for the reduction of graphene oxide by sodium borohydride with CaCl2 as a catalyst. New Carbon Mater 30(1):41–47. https://doi.org/10.1016/S1872-5805(15)60174-3
Yang S, Zhi L, Tang K, Feng X, Müllen K (2012) Efficient synthesis of heteroatom (N or S)-doped graphene based on ultrathin graphene oxide-porous silica sheets for oxygen reduction reactions. Adv Funct Mater 22(17):3634–3640. https://doi.org/10.1002/adfm.201200186
Wang X, Qin Y, Zhu L, Tang H (2015) Nitrogen-doped reduced graphene oxide as a bifunctional material for removing bisphenols: synergistic effect between adsorption and catalysis. Environ Sci Technol 49(11):6855–6864. https://doi.org/10.1021/acs.est.5b01059
Panda D, Nandi A, Datta SK, Saha H, Majumdar S (2016) Selective detection of carbon monoxide (CO) gas by reduced graphene oxide (rGO) at room temperature. RSC Adv 6(53):47337–47348. https://doi.org/10.1039/c6ra06058g
Melios C, Giusca CE, Panchal V, Kazakova O (2018) Water on graphene: review of recent progress. 2D Materials 5(2):022001. https://doi.org/10.1088/2053-1583/aa9ea9
Lounasvuori MM, Rosillo-Lopez M, Salzmann CG, Caruana DJ, Holt KB (2014) Electrochemical characterisation of graphene nanoflakes with functionalised edges. Faraday Discuss 172:293–310. https://doi.org/10.1039/c4fd00034j
Basnig D, Vilá N, Herzog G, Walcarius A (2020) Voltammetric behaviour of cationic redox probes at mesoporous silica film electrodes. J Electroanal Chem. https://doi.org/10.1016/j.jelechem.2020.113993
Rose A, Raghavan N, Thangavel S, Uma Maheswari B, Nair DP, Venugopal G (2015) Investigation of cyclic voltammetry of graphene oxide/polyaniline/polyvinylidene fluoride nanofibers prepared via electrospinning. Mater Sci Semicond Process 31:281–286. https://doi.org/10.1016/j.mssp.2014.10.051
Hsine Z, Bizid S, Mlika R, Sauriat-Dorizon H, Haj Said A, Korri-Youssoufi H (2020) Nanocomposite based on poly (para-phenylene)/chemical reduced graphene oxide as a platform for simultaneous detection of ascorbic acid, dopamine and uric acid. Sensors 20(5):1256. https://doi.org/10.3390/s20051256
Jeon W-Y, Lee C-J, Sut TN, Kim H-H, Choi Y-B (2021) Pentacyanoammineferrate-based non-enzymatic electrochemical biosensing platform for selective uric acid measurement. Sensors 21:1574. https://doi.org/10.3390/s21051574
Walter GW (1986) A review of impedance plot methods used for corrosion performance analysis of painted metals. Corros Sci 26(9):681–703. https://doi.org/10.1016/0010-938X(86)90033-8. (Printed in Great Britain)
Jiang J, Du X (2014) Sensitive electrochemical sensors for simultaneous determination of ascorbic acid, dopamine, and uric acid based on Au@Pd-reduced graphene oxide nanocomposites. Nanoscale 6(19):11303–11309. https://doi.org/10.1039/c4nr01774a
Hsine Z, Blili S, Milka R, Dorizon H, Said AH, Korri-Youssoufi H (2020) Sensor based on redox conjugated poly(para-phenylene) for the simultaneous detection of dopamine, ascorbic acid, and uric acid in human serum sample. Anal Bioanal Chem 412(18):4433–4446. https://doi.org/10.1007/s00216-020-02686-6
Yang YJ (2015) One-pot synthesis of reduced graphene oxide/zinc sulfide nanocomposite at room temperature for simultaneous determination of ascorbic acid, dopamine and uric acid. Sens Actuators, B Chem 221:750–759. https://doi.org/10.1016/j.snb.2015.06.150
Xue C, Li H, An H, Yang B, Wei J, Yang G (2018) Quantum dots accelerate electrons transfer in Cd0.8Zn0.2S photocatalytic system via rGO nanosheet “bridge” towards visible-light-driven hydrogen evolution. ACS Catal. https://doi.org/10.1021/acscatal.7b04228
Raj MA, John SA (2013) Simultaneous determination of uric acid, xanthine, hypoxanthine and caffeine in human blood serum and urine samples using electrochemically reduced graphene oxide modified electrode. AnalyticaChimicaActa 771:14–20. https://doi.org/10.1016/j.aca.2013.02.017
Sakthinathan S, Keyan AK, Rajakumaran R, Chen S-M, Chiu T-W, Dong C, Vinothini S (2021) Synthesis of N-rGO-MWCNT/CuCrO2 catalyst for the bifunctional application of hydrogen evolution reaction and electrochemical detection of Bisphenol-A. Catalysts 11:301. https://doi.org/10.3390/catal11030301
Motshakeri M, Phillips ARJ, Kilmartin PA (2019) Application of cyclic voltammetry to analyse uric acid and reducing agents in commercial milks. Food Chem 293:23–31. https://doi.org/10.1016/j.foodchem.2019.04.0
Alonso-Lomillo MA, Domínguez-Renedo O, Saldaña-Botín A, Arcos-Martínez MJ (2017) Determination of ascorbic acid in serum samples by screen-printed carbon electrodes modified with gold nanoparticles. Talanta 174:733–737. https://doi.org/10.1016/j.talanta.2017.07.015
Wang Z, Guo H, Gui R, Jin H, Xia J, Zhang F (2018) Simultaneous and selective measurement of dopamine and uric acid using glassy carbon electrodes modified with a complex of gold nanoparticles and multiwall carbon nanotubes. Sens Actuators, B Chem 255:2069–2077. https://doi.org/10.1016/j.snb.2017.09.010
Yan Q, Zhi N, Yang L, Xu G, Feng Q, Zhang Q, Sun S (2020) A highly sensitive uric acid electrochemical biosensor based on a nano-cube cuprous oxide/ferrocene/uricase modified glassy carbon electrode. Sci Rep. https://doi.org/10.1038/s41598-020-67394-8
Azzara F, Patella B, Aiello G, O’Riordan A, Torino C, Vilasi A, Inguanta R (2021) Electrochemical detection of uric acid and ascorbic acid using r-GO/NPs based sensors. ElectrochimicaActa 388:138652. https://doi.org/10.1016/j.electacta.2021.1386
Mazzara F, Patella B, Aiello G, O’Riordan A, Torino C, Vilasi A, Inguanta R (2021) Electrochemical detection of uric acid and ascorbic acid using r-GO/NPs based sensors. ElectrochimicaActa 388:138652. https://doi.org/10.1016/j.electacta.2021.138652
Aparna TK, Sivasubramanian R, Dar MA (2018) One-pot synthesis of Au-Cu2O/rGO nanocomposite based electrochemical sensor for selective and simultaneous detection of dopamine and uric acid. J Alloys Compd 741:1130–1141. https://doi.org/10.1016/J.JALLCOM.2018.01.205
Ghanbari K, Moloudi M (2016) Flower-like ZnO decorated polyaniline/reduced graphene oxide nanocomposites for simultaneous determination of dopamine and uric acid. Anal Biochem 512:91–102. https://doi.org/10.1016/j.ab.2016.08.014
Kogularasu S, Akilarasan M, Chen SM, Chen TW, Lou BS (2019) Urea-based morphological engineering of ZnO; for the biosensing enhancement towards dopamine and uric acid in food and biological samples. Mater Chem Phys 227:5–11. https://doi.org/10.3390/nano9060835
Acknowledgements
The authors thank Mrs. Hafsa Korry-youssefi CNRS Research Director at the University Paris Saclay (France) for provinding the gaphene oxide and Dr Wajdi Blekacem, Assistant Professor at the Faculty of Science of Monastir, for the XPS measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Besbes, F., Hsine, Z. & Mlika, R. Synthesis of heteroatoms doped reduced graphene oxide for the electrochemical determination of uric acid in commercial milk. Carbon Lett. 33, 2109–2128 (2023). https://doi.org/10.1007/s42823-023-00552-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-023-00552-w