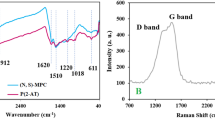
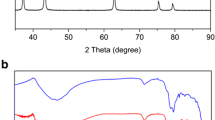
Abstract
The disposal of organic pollutants is one of the important research topics. Some of the studies in this field are based on the degradation of organic pollutants with a catalytic agent. The cobalt tetraoxide/peroxymonosulfate system is an important catalytic system used for the radical degradation of organic pollutants. To increase the catalytic efficiency of such reactions, graphitization of activated carbon used as a support solid and nitrogen doping to the carbon structure are commonly used methods. In this study, cobalt tetraoxide production, N-doping and graphitization were carried out in a single step by heat treatment of activated carbon doped with the phthlocyanine cobalt (II) complex. The catalytic performance of the catalyst/peroxymonosulfate system was investigated by changing the pH, catalyst, and PMS concentration parameters on rhodamine B and 1,3,5 trichlorophenol, which were used as models. It was seen that the catalysts had 97% activity on rhodamine B in 16 min and 100% on 1,3,5 trichlorophenol in 6 min. It was observed that the catalysts continued to show high catalytic activity for five cycles in reusability studies and had a very low cobalt leaching rate. These results are in good agreement with previously published studies. In line with these results, the synthesized N-doped graphitic carbon/Co3O4 catalyst can be used as an effective catalyst for wastewater treatments.
Graphical abstract














Similar content being viewed by others
Abbreviations
- Co3O4:
-
Cobaltite
- AC:
-
Activated carbon
- RhB:
-
Rhodamine B
- 2,4,6-TCP:
-
2,4,6-Trichlorophenol
- PMS:
-
Peroxymonosulfate
- PS:
-
Persulfate
- XRD:
-
X-ray powder diffraction
- SEM:
-
Scanning electron microscope
- FTIR:
-
Fourier transform-infrared
- BET:
-
Brunauer–Emmett–Teller
- eV:
-
Electron volt
- OH·:
-
Hydroxyl radical
- SO4˙ ¯:
-
Sulfate radical
- O2˙ ¯:
-
Peroxide radical
- 1O2 :
-
Singlet oxygen
- Co:
-
Cobalt
- TEM:
-
Transmission electron microscopy
- THF:
-
Tetrahydrofuran
- TBA:
-
tert-Butyl alcohol
- p-BQ:
-
p-Benzoquinone
- QS-DFT:
-
Quenched solid density functional theory
- BJH:
-
Barret–Joyner–Halende
- N2 :
-
Nitrogen
- nm:
-
Nanometer
- Co :
-
Initial concentration
- Ct :
-
Concentration at time
- k:
-
Fisrt-order reaction constant
- EtOH:
-
Ethanol
- TOC:
-
Total organic carbon
References
Pearce CI, Lloyd JR, Guthrie JT (2003) The removal of colour from textile wastewater using whole bacterial cells: a review. Dye Pigment 58:179–196
Roberts ALK, Fletcher JM, Moore L, Byers S (2010) Trans-generational exposure to low levels of rhodamine B does not adversely affect litter size or liver function in murine mucopolysaccharidosis type IIIA. Mol Genet Metab 101:208–213. https://doi.org/10.1016/j.ymgme.2010.06.008
Dai Z, Ren PG, Zhang H et al (2021) Nitrogen-doped and hierarchically porous carbon derived from spent coffee ground for efficient adsorption of organic dyes. Carbon Lett 31:1249–1260. https://doi.org/10.1007/s42823-021-00248-z
Jin T, Liu C, Chen F et al (2022) Synthesis of g-C3N4/CQDs composite and its photocatalytic degradation property for Rhodamine B. Carbon Lett 32:1451–1462. https://doi.org/10.1007/s42823-022-00382-2
Li H, Kuang X, Shen X, Zhu J (2020) Comparative electrochemical oxidation of the secondary effluent of petrochemical wastewater with electro-fenton and anodic oxidation with supporting electrolytes. Environ Technol (United Kingdom) 43:1–24. https://doi.org/10.1080/09593330.2020.1791971
Rakhmania A, Kamyab H, Yuzir MA et al (2022) Electrochemical oxidation of palm oil mill effluent using platinum as anode: optimization using response surface methodology. Environ Res 214:113993. https://doi.org/10.1016/j.envres.2022.113993
Hazarika KK, Hazarika D, Bharali P (2020) Binary α-Fe2O3–Co3O4 nanostructures for advanced oxidation process: role of synergy for enhanced catalysis. Appl Organomet Chem 34:1–11. https://doi.org/10.1002/aoc.5920
Qin FX, Jia SY, Liu Y et al (2013) Metal-organic framework as a template for synthesis of magnetic CoFe 2O4 nanocomposites for phenol degradation. Mater Lett 101:93–95. https://doi.org/10.1016/j.matlet.2013.03.085
Jiang Y, Wan S, Zhao W et al (2022) Reusable, magnetic laser-induced graphene for efficient removal of organic pollutants from water. Carbon Lett 32:1047–1064. https://doi.org/10.1007/s42823-022-00336-8
Yang S, Li L, Xiao T et al (2017) Reuse performance of granular-activated carbon and activated carbon fiber in catalyzed peroxymonosulfate oxidation. Environ Technol (United Kingdom) 38:598–605. https://doi.org/10.1080/09593330.2016.1205147
Sudhaik A, Raizada P, Thakur S et al (2020) Metal-free photo-activation of peroxymonosulfate using graphene supported graphitic carbon nitride for enhancing photocatalytic activity. Mater Lett 277:128277. https://doi.org/10.1016/j.matlet.2020.128277
Ramesh M, Rajeshkumar L, Bhoopathi R (2021) Carbon substrates: a review on fabrication, properties and applications. Carbon Lett 31:557–580. https://doi.org/10.1007/s42823-021-00264-z
Du J, Xu W, Liu J, Zhao Z (2020) Efficient degradation of Acid Orange 7 by persulfate activated with a novel developed carbon-based MnFe2O4 composite catalyst. J Chem Technol Biotechnol 95:1135–1145. https://doi.org/10.1002/jctb.6298
Ren Z, Romar H, Varila T et al (2021) Ibuprofen degradation using a Co-doped carbon matrix derived from peat as a peroxymonosulphate activator. Environ Res 193:110564. https://doi.org/10.1016/j.envres.2020.110564
Xu H, Zhang Y, Li J et al (2020) Heterogeneous activation of peroxymonosulfate by a biochar-supported Co3O4 composite for efficient degradation of chloramphenicols. Environ Pollut 257:113610. https://doi.org/10.1016/j.envpol.2019.113610
Zhao DY, Wang HL, Qi HP, Zhao Y (2019) Facile synthesis of mesoporous Co3O4 with excellent performance for activation of PMS. Mater Res Express. https://doi.org/10.1088/2053-1591/ab1686
Zeng T, Zhang H, He Z et al (2016) Mussel-inspired approach to constructing robust cobalt-embedded N-doped carbon nanosheet toward enhanced sulphate radical-based oxidation. Sci Rep 6:2–10. https://doi.org/10.1038/srep33348
Hu Y, Chen D, Wang S et al (2022) Activation of peroxymonosulfate by nitrogen-doped porous carbon for efficient degradation of organic pollutants in water: performance and mechanism. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2021.119791
Kwiatkowski M, Broniek E (2020) An evaluation of the reliability of the results obtained by the LBET, QSDFT, BET, and DR methods for the analysis of the porous structure of activated carbons. Materials (Basel). https://doi.org/10.3390/MA13183929
Ghani F, Kristen J, Riegler H (2012) Solubility properties of unsubstituted metal phthalocyanines in different types of solvents. J Chem Eng Data 57:439–449. https://doi.org/10.1021/je2010215
Sundar M, Easwaramoorthy D, Kutti Rani S, Mohammed Bilal I (2008) Mn(II) catalysed decomposition of peroxomonosulphate—Kinetic and mechanistic study. Catal Commun 9:2340–2344. https://doi.org/10.1016/j.catcom.2008.05.024
Rasalingam S, Wu CM, Koodali RT (2015) Modulation of pore sizes of titanium dioxide photocatalysts by a facile template free hydrothermal synthesis method: Implications for photocatalytic degradation of rhodamine B. ACS Appl Mater Interfaces 7:4368–4380. https://doi.org/10.1021/am508883f
Bisbyb RH, Morgan CG, Hamblett I, Gorman AA (1999) Quenching of Singlet Oxygen by Trolox C, Ascorbate, and Amino Acids: Effects of pH and Temperature. J Phys Chem A 103:7454–7459. https://doi.org/10.1021/jp990838c
Khan A, Zou S, Wang T et al (2018) Facile synthesis of yolk shell Mn2O3@Mn5O8 as an effective catalyst for peroxymonosulfate activation. Phys Chem Chem Phys 20:13909–13919. https://doi.org/10.1039/c8cp02080a
Qin Q, Gao X, Wu X, Liu Y (2019) NaBH4-treated cobalt-doped g-C3N4 for enhanced activation of peroxymonosulfate. Mater Lett 256:126623. https://doi.org/10.1016/j.matlet.2019.126623
Kang M, Zhou H (2015) Facile synthesis and structural characterization of Co3O4 nanocubes. AIMS Mater Sci 2:16–27. https://doi.org/10.3934/matersci.2015.1.16
Üner O, Bayrak Y (2018) The effect of carbonization temperature, carbonization time and impregnation ratio on the properties of activated carbon produced from Arundo donax. Microporous Mesoporous Mater 268:225–234. https://doi.org/10.1016/j.micromeso.2018.04.037
Kumar A, Jena HM (2016) Preparation and characterization of high surface area activated carbon from Fox nut (Euryale ferox) shell by chemical activation with H3PO4. Results Phys 6:651–658. https://doi.org/10.1016/j.rinp.2016.09.012
Aluha J, Abatzoglou N, Gitzhofer F Production of active carbon-supported Fischer-Tropsch nano-catalysts using induction suspension plasma-spray (SPS) technology
Le Van K, Luong TTT (2019) Preparation of pore-size controllable activated carbon from rice husk using dual activating agent and its application in supercapacitor. J Chem. https://doi.org/10.1155/2019/4329609
Ghanbari F, Moradi M (2017) Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: review. Chem Eng J 310:41–62
Wang YR, Chu W (2011) Degradation of a xanthene dye by Fe(II)-mediated activation of Oxone process. J Hazard Mater 186:1455–1461. https://doi.org/10.1016/j.jhazmat.2010.12.033
Zhang Y, Huo JB, Yang JCE, Fu ML (2019) Facile fabrication of elastic CoO@graphene aerogel for recycled degradation of chloramphenicol. Mater Lett 240:88–91. https://doi.org/10.1016/j.matlet.2018.12.132
Anipsitakis GP, Stathatos E, Dionysiou DD (2005) Heterogeneous activation of Oxone using Co 3O 4. J Phys Chem B 109:13052–13055. https://doi.org/10.1021/jp052166y
Yang Y, Jiang J, Lu X et al (2015) Production of sulfate radical and hydroxyl radical by reaction of ozone with peroxymonosulfate: a novel advanced oxidation process. Environ Sci Technol 49:73307339. https://doi.org/10.1021/es506362e
Yuan R, Ramjaun SN, Wang Z, Liu J (2011) Effects of chloride ion on degradation of Acid Orange 7 by sulfate radical-based advanced oxidation process: Implications for formation of chlorinated aromatic compounds. J Hazard Mater 196:173–179. https://doi.org/10.1016/j.jhazmat.2011.09.007
Antoniou MG, de la Cruz AA, Dionysiou DD (2010) Degradation of microcystin-LR using sulfate radicals generated through photolysis, thermolysis and e- transfer mechanisms. Appl Catal B Environ 96:290–298. https://doi.org/10.1016/j.apcatb.2010.02.013
Ling SK, Wang S, Peng Y (2010) Oxidative degradation of dyes in water using Co2+/H2O2 and Co2+/peroxymonosulfate. J Hazard Mater 178:385–389. https://doi.org/10.1016/j.jhazmat.2010.01.091
Huang YF, Huang YH (2009) Behavioral evidence of the dominant radicals and intermediates involved in Bisphenol A degradation using an efficient Co2+/PMS oxidation process. J Hazard Mater 167:418–426. https://doi.org/10.1016/j.jhazmat.2008.12.138
Pang Y, Kong L, Chen D et al (2020) Facilely synthesized cobalt doped hydroxyapatite as hydroxyl promoted peroxymonosulfate activator for degradation of Rhodamine B. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2019.121447
Dung NT, Thu TV, Van Nguyen T et al (2020) Catalytic activation of peroxymonosulfate with manganese cobaltite nanoparticles for the degradation of organic dyes. RSC Adv 10:3775–3788. https://doi.org/10.1039/c9ra10169a
Li H, Wan J, Ma Y et al (2016) Degradation of refractory dibutyl phthalate by peroxymonosulfate activated with novel catalysts cobalt metal-organic frameworks: Mechanism, performance, and stability. J Hazard Mater 318:154–163. https://doi.org/10.1016/j.jhazmat.2016.06.058
Erdem H, Erdem M (2020) Synthesis and characterization of a novel activated carbon–supported cobalt catalyst from biomass mixture for tetracycline degradation via persulfate activation. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00963-z
Mahamallik P, Pal A (2020) Photo-Fenton process in Co(II)-adsorbed admicellar soft-template on alumina support for methyl orange degradation. Catal Today 348:212–222. https://doi.org/10.1016/j.cattod.2019.07.045
Chen L, Yang S, Zuo X et al (2018) Biochar modification significantly promotes the activity of Co3O4 towards heterogeneous activation of peroxymonosulfate. Chem Eng J 354:856–865. https://doi.org/10.1016/j.cej.2018.08.098
Pi Y, Gao H, Cao Y et al (2020) Cobalt ferrite supported on carbon nitride matrix prepared using waste battery materials as a peroxymonosulfate activator for the degradation of levofloxacin hydrochloride. Chem Eng J. https://doi.org/10.1016/j.cej.2019.122377
Kang S, Hwang J (2021) CoMn2O4 embedded hollow activated carbon nanofibers as a novel peroxymonosulfate activator. Chem Eng J. https://doi.org/10.1016/j.cej.2020.127158
Li L, Wu H, Chen H et al (2020) Heterogeneous activation of peroxymonosulfate by hierarchically porous cobalt/iron bimetallic oxide nanosheets for degradation of phenol solutions. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.127160
Ghanbari F, Moradi M, Gohari F (2016) Degradation of 2,4,6-trichlorophenol in aqueous solutions using peroxymonosulfate/activated carbon/UV process via sulfate and hydroxyl radicals. J Water Process Eng 9:22–28. https://doi.org/10.1016/j.jwpe.2015.11.011
Günay T, Çimen Y (2017) Degradation of 2,4,6-trichlorophenol with peroxymonosulfate catalyzed by soluble and supported iron porphyrins. Environ Pollut 231:1013–1020. https://doi.org/10.1016/j.envpol.2017.08.059
Tabit R, Amadine O, Essamlali Y et al (2018) Magnetic CoFe2O4 nanoparticles supported on graphene oxide (CoFe2O4/GO) with high catalytic activity for peroxymonosulfate activation and degradation of rhodamine B. RSC Adv 8:1351–1360. https://doi.org/10.1039/c7ra09949e
Zhu J, Zhu Z, Zhang H et al (2019) Calcined CoAl-layered double hydroxide as a heterogeneous catalyst for the degradation of acetaminophen and rhodamine B: activity, stability, and mechanism. Environ Sci Pollut Res 26:33329–33340. https://doi.org/10.1007/s11356-019-06390-6
Huang C, Wang Y, Gong M et al (2020) Α-MnO2/Palygorskite composite as an effective catalyst for heterogeneous activation of peroxymonosulfate (PMS) for the degradation of Rhodamine B. Sep Purif Technol 230:115877. https://doi.org/10.1016/j.seppur.2019.115877
Zhao L, Yang D, Ma L et al (2021) An efficient heterogeneous catalyst of FeCo2O4/g-C3N4 composite for catalytic peroxymonosulfate oxidation of organic pollutants under visible light. Colloids Surf A Physicochem Eng Asp. https://doi.org/10.1016/j.colsurfa.2020.125725
Zhang D, Li Y, Guo J et al (2021) MOFs-derived magnetic C@Cu–Ni bimetal particles: an efficient peroxymonosulfate activator for 2,4,6-trichlorophenol degradation. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.129394
Zhu B, Yu Y, Ding Y, Ge S (2022) Iron-modified granular sludge biochar-based catalysts for improved Rhodamine B degradation by activating peroxymonosulfate. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-03340-0
Chen S, Hu J, Lu L et al (2022) Iron porphyrin-TiO2 modulated peroxymonosulfate activation for efficient degradation of 2,4,6-trichlorophenol with high-valent iron-oxo species. Chemosphere 309:136744. https://doi.org/10.1016/j.chemosphere.2022.136744
Wang A, Ni J, Wang W et al (2022) MOF derived Co−Fe nitrogen doped graphite carbon@crosslinked magnetic chitosan Micro−nanoreactor for environmental applications: Synergy enhancement effect of adsorption−PMS activation. Appl Catal B Environ 319:121926. https://doi.org/10.1016/j.apcatb.2022.121926
Wang M, Li T, Hou Q et al (2022) Facile one-step preparation of Co and Ce doped TiO2 in visible light PMS activation for PAHs degradation. Chemosphere 308:136360. https://doi.org/10.1016/j.chemosphere.2022.136360
Li Y, Zhang S, Qin Y et al (2022) Preparation of cobalt/hydrochar using the intrinsic features of rice hulls for dynamic carbamazepine degradation via efficient PMS activation. J Environ Chem Eng 10:108659. https://doi.org/10.1016/j.jece.2022.108659
Wang S, Wang J (2022) Bimetallic and nitrogen co-doped biochar for peroxymonosulfate (Pms) activation to degrade emerging contaminants. SSRN Electron J. https://doi.org/10.2139/ssrn.4273735
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no potential conflicts of interest relevant to this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akçay, H.T., Demir, A., Özçifçi, Z. et al. Treatment of wastewater containing organic pollutants in the presence of N-doped graphitic carbon and Co3O4/peroxymonosulfate. Carbon Lett. 33, 1445–1460 (2023). https://doi.org/10.1007/s42823-022-00452-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-022-00452-5