Abstract
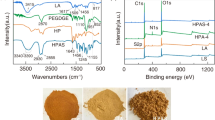
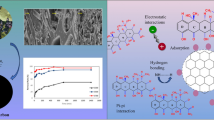
Here, a novel nitrogen-doped carbon nano-material (N-CGNM) with hierarchically porous structure was prepared from spent coffee ground for efficient adsorption of organic dyes by a simple one-step carbonization process (the uniform mixture consists of spent coffee ground, urea, and CaCl2 with the ratio of 1:1:1, which was heated to 1000 °C with a rate of 10 °C min−1 and held at 1000 °C for 90 min in N2 atmosphere to carry out carbonization, activation, and N-doping concurrently). The morphology and structure analysis show that the prepared N-CGNM exhibits hierarchical pore structure, high specific surface area (544 m2/g), and large numbers of positively charged nitrogen-containing groups. This unique structure and chemical composition endow N-CGNM with an excellent adsorption capacity toward anion Congo red (623.12 ± 21.69 mg/g), which is obviously superior to that (216.47 ± 18.43 mg/g) of untreated spent coffee ground-based carbon nano-materials (CGM). Oppositely, the adsorption capacity of N-CGNM towards cation methylene blue is inferior to that of CGM due to the existence of electrostatic repulsion. These findings show a great guidance for the development of low-cost but efficient selective adsorbent.








Similar content being viewed by others
References
Ali H (2010) Biodegradation of Synthetic Dyes—A Review. Water Air Soil Pollut 213:251–273
Khaled A, El Nemr A, El-Sikaily A, Abdelwahab O (2009) Removal of Direct N Blue-106 from artificial textile dye effluent using activated carbon from orange peel: adsorption isotherm and kinetic studies. J Hazard Mater 165:100–110
Qu X, Alvarez PJ, Li Q (2013) Applications of nanotechnology in water and wastewater treatment. Water Res 47:3931–3946
Tang R, Wang YL, Yuan SJ, Wang W, Yue Z, Zhan XM, Hu ZH (2020) Organoarsenic feed additives in biological wastewater treatment processes: removal, biotransformation, and associated impacts. J Hazard Mater 406:124789
El-Gohary F, Tawfik A (2009) Decolorization and COD reduction of disperse and reactive dyes wastewater using chemical-coagulation followed by sequential batch reactor (SBR) process. Desalination 249:1159–1164
Wu T, Cai X, Tan S, Li H, Liu J, Yang W (2011) Adsorption characteristics of acrylonitrile, p-toluenesulfonic acid, 1-naphthalenesulfonic acid and methyl blue on graphene in aqueous solutions. Chem Eng J 173:144–149
Wong S, Ngadi N, Inuwa IM, Hassan O (2018) Recent advances in applications of activated carbon from bio-waste for wastewater treatment: a short review. J Clean Prod 175:361–375
Dong Z, Wang D, Liu X, Pei X, Chen L, Jin J (2014) Bio-inspired surface-functionalization of graphene oxide for the adsorption of organic dyes and heavy metal ions with a superhigh capacity. Journal of Materials Chemistry A 2:5034–5040
Yi FY, Zhu W, Dang S, Li JP, Wu D, Li YH, Sun ZM (2015) Polyoxometalates-based heterometallic organic-inorganic hybrid materials for rapid adsorption and selective separation of methylene blue from aqueous solutions. Chem Commun 51:3336–3339
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Coll Interface Sci 209:172–184
Dai Y, Zhang N, Xing C, Cui Q, Sun Q (2019) The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: a review. Chemosphere 223:12–27
Menya E, Olupot PW, Storz H, Lubwama M, Kiros Y (2018) Production and performance of activated carbon from rice husks for removal of natural organic matter from water: a review. Chem Eng Res Des 129:271–296
Yusuf M, Elfghi FM, Zaidi SA, Abdullah EC, Khan MA (2015) Applications of graphene and its derivatives as an adsorbent for heavy metal and dye removal: a systematic and comprehensive overview. RSC Adv 5:50392–50420
Jain A, Balasubramanian R, Srinivasan MP (2016) Hydrothermal conversion of biomass waste to activated carbon with high porosity: a review. Chem Eng J 283:789–805
Bhatnagar A, Sillanpää M, Krowiak AW (2015) Agricultural waste peels as versatile biomass for water purification—a review. Chem Eng J 270:244–271
Arias JO, Arias SD, Correa YD, Avila EL, MontaNo D, Simpson R, Castro OV (2020) New powder material obtained from spent coffee ground and whey protein; Thermal and morphological analysis. Mater Chem Phys 240:122171
Cloete KJ, Smit Z, Ndimba RM, Vavpeti P, Plessis AD, Roux SGL, Pelicon P (2019) Physico-elemental analysis of roasted organic coffee beans from Ethiopia, Colombia, Honduras, and Mexico using X-ray micro-computed tomography and external beam particle induced X-ray emission. Food Chem 2:100032
Nguyen VT, Nguyen TB, Chen CW, Hung CM, Chang JH, Dong CD (2019) Influence of pyrolysis temperature on polycyclic aromatic hydrocarbons production and tetracycline adsorption behavior of biochar derived from spent coffee ground. Biores Technol 284:197–203
Yahya MA, Al-Qodah Z, Ngah CWZ (2015) Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: a review. Renew Sustain Energy Rev 46:218–235
Chen Y, Zhu Y, Wang Z, Li Y, Wang L, Ding L, Gao X, Ma Y, Guo Y (2011) Application studies of activated carbon derived from rice husks produced by chemical-thermal process—a review. Adv Coll Interface Sci 163:39–52
Liou TH (2010) Development of mesoporous structure and high adsorption capacity of biomass-based activated carbon by phosphoric acid and zinc chloride activation. Chem Eng J 158:129–142
Williams PT, Reed AR (2004) High grade activated carbon matting derived from the chemical activation and pyrolysis of natural fibre textile waste. J Anal Appl Pyrol 71:971–986
Kundu A, Mondal A (2019) Kinetics, isotherm, and thermodynamic studies of methylene blue selective adsorption and photocatalysis of malachite green from aqueous solution using layered Na-intercalated Cu-doped Titania. Appl Clay Sci 183:105323
Li M, Zhao H, Lu ZY (2020) Porphyrin-based porous organic polymer, Py-POP, as a multifunctional platform for efficient selective adsorption and photocatalytic degradation of cationic dyes. Microporous Mesoporous Mater 292:109774
Lin R, Liang Z, Yang C, Shi W, Cui F, Zhao Z (2019) Selective and enhanced adsorption of the monosubstituted benzenes on the Fe-modified MCM-41: contribution of the substituent groups. Chemosphere 237:124546
Wang W, Xu L, Li X, Yang Y, An E (2014) Self-healing properties of protective coatings containing isophorone diisocyanate microcapsules on carbon steel surfaces. Corros Sci 80:528–535
Allan D, Daly JH, Liggat JJ (2019) Oxidative and non-oxidative degradation of a TDI-based polyurethane foam: Volatile product and condensed phase characterisation by FTIR and solid state 13C NMR spectroscopy. Polym Degrad Stab 161:57–73
Ou Y, Sun Y, Guo X, Jiao Q (2018) Investigation on the thermal decomposition of hydroxyl terminated polyether based polyurethanes with inert and energetic plasticizers by DSC-TG-MS-FTIR. J Anal Appl Pyrol 132:94–101
Zhao G, Chen C, Yu D, Sun L, Yang C, Zhang H, Sun Y, Besenbacher F, Yu M (2018) One-step production of O-N-S co-doped three-dimensional hierarchical porous carbons for high-performance supercapacitors. Nano Energy 47:547–555
Peng H, Ma G, Sun K, Zhang Z, Yang Q, Lei Z (2016) Nitrogen-doped interconnected carbon nanosheets from pomelo mesocarps for high performance supercapacitors. Electrochim Acta 190:862–871
Li Y, Wang G, Wei T, Fan Z, Yan P (2016) Nitrogen and sulfur co-doped porous carbon nanosheets derived from willow catkin for supercapacitors. Nano Energy 19:165–175
Puziy AM, Poddubnaya OI, Socha RP, Gurgul J, Wisniewski M (2008) XPS and NMR studies of phosphoric acid activated carbons. Carbon 46:2113–2123
Mungse HP, Gupta K, Singh R, Sharma OP, Sugimura H, Khatri OP (2019) Alkylated graphene oxide and reduced graphene oxide: Grafting density, dispersion stability to enhancement of lubrication properties. J Colloid Interface Sci 541:150–162
Chen Y, Liu Z, Sun L, Lu Z, Zhuo K (2018) Nitrogen and sulfur co-doped porous graphene aerogel as an efficient electrode material for high performance supercapacitor in ionic liquid electrolyte. J Power Sources 390:215–223
Wang N, Song H, Ren H, Chen J, Yao M, Huang W, Hu W, Komarneni S (2019) Partly nitrogenized nickel oxide hollow spheres with multiple compositions for remarkable electrochemical performance. Chem Eng J 358:531–539
Xin G, Wang M, Zhang W, Song J, Zhang B (2018) Preparation of high-capacitance N, S co-doped carbon nanospheres with hierarchical pores as supercapacitors. Electrochim Acta 291:168–176
Dai Z, Ren P-G, Jin Y-L, Zhang H, Ren F, Zhang Q (2019) Nitrogen-sulphur Co-doped graphenes modified electrospun lignin/polyacrylonitrile-based carbon nanofiber as high performance supercapacitor. J Power Sources 437:226937
Dai Z, Shi X, Liu H, Li H, Han Y, Zhou J (2018) High-strength lignin-based carbon fibers via a low-energy method. RSC Adv 8:1218–1224
Chen J, Fang K, Chen Q, Xu J, Wong C-P (2018) Integrated paper electrodes derived from cotton stalks for high-performance flexible supercapacitors. Nano Energy 53:337–344
Yang S, Wang S, Liu X, Li L (2019) Biomass derived interconnected hierarchical micro-meso-macro- porous carbon with ultrahigh capacitance for supercapacitors. Carbon 147:540–549
Su P, Jiang L, Zhao J, Yan J, Li C, Yang Q (2012) Mesoporous graphitic carbon nanodisks fabricated via catalytic carbonization of coordination polymers. Chem Commun 48:8769
Peng H, Ma G, Sun K, Mu J, Lei Z (2014) One-step preparation of ultrathin nitrogen-doped carbon nanosheets with ultrahigh pore volume for high-performance supercapacitors. Journal of Materials Chemistry A 2:17297–17301
Tiwari JN, Mahesh K, Le NH, Kemp KC, Timilsina R, Tiwari RN, Kim KS (2013) Reduced graphene oxide-based hydrogels for the efficient capture of dye pollutants from aqueous solutions. Carbon 56:173–182
Xu J, Wang L, Zhu Y (2012) Decontamination of bisphenol a from aqueous solution by graphene adsorption. Langmuir 28:8418–8425
Roy A, Adhikari B, Majumder SB (2013) Equilibrium, kinetic, and thermodynamic studies of azo dye adsorption from aqueoussolution by chemically modified lignocellulosic jute fiber. Ind Eng Chem Res 52:6502–6512
Huang X, Liao X, Shi B (2009) Hg(II) removal from aqueous solution by bayberry tannin-immobilized collagen fiber. J Hazard Mater 170:1141–1148
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 51773167, 21706208, and 51573147) and the Science and Technology Plan Project of Xi'an(2019217814GXRC014CG015-GXYD14.8).
Author information
Authors and Affiliations
Contributions
ZD: conceptualization, methodology, writing—original draft. P-GR: resources, writing—review & editing, and supervision. HZ: software and data curation. XG: validation. Y-LJ: resources, writing—review & editing, and supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dai, Z., Ren, PG., Zhang, H. et al. Nitrogen-doped and hierarchically porous carbon derived from spent coffee ground for efficient adsorption of organic dyes. Carbon Lett. 31, 1249–1260 (2021). https://doi.org/10.1007/s42823-021-00248-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-021-00248-z