Abstract
Biochar applications have an enormous impact on the soil microbial community and functionality. However, the majority of the knowledge on biochar–microbe interaction derives almost exclusively from bacterial and fungal studies, while the vast majority of eukaryotic diversity, protists, are mostly neglected. Protists play important roles in the soil ecosystem as microbial predators, decomposers, photoautotrophs, pathogens, and parasites and they are essential for a healthy soil ecosystem. Toward a comprehensive understanding of the effects of biochar application, we need more studies on protists across the full breadth of eukaryotic diversity. The aim of this article is to highlight the research needs and discuss potential research ideas on biochar–protist interaction, which would advance our knowledge of biochar–microbe interaction.
Highlights
-
Biochar–microbe interaction is almost exclusively studied for bacteria and fungi.
-
Only a few studies are available on how soil protists react to biochar application.
-
More research on biochar–protist is needed for a better understanding of biochar–microbe interaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Protists, any eukaryotic organism that is not a plant, fungi, or animal, are a major group in the soil microbiome (Geisen et al. 2018; Chandarana and Amaresan 2022). Their enormous taxonomic diversity reflects immense functionalities. The major functional group of protists is the predators, representing more than half of the protist diversity (Gao et al. 2019). The predatory protists feed on other organisms, including bacteria, archaea, fungi, and nematodes, and thus, control soil biodiversity and their population, stimulate microbial activity, and substantially contribute to nutrient cycling and plant productivity (Geisen et al. 2018; Gao et al. 2019; Xiong et al. 2020; Guo et al. 2021; Chandarana and Amaresan 2022). Decomposer protists play crucial roles in nutrient cycling via organic matter degradation (Geisen et al. 2018). Protists include photoautotrophic organisms, which are major players in the global soil carbon balance. For instance, carbon fixed by the soil algae corresponds to about 6% of the net primary production of the whole terrestrial vegetation (Jassey et al. 2022). Not all soil protists are beneficial as plant pathogenic protists (mainly belonging to Oomycetes) cause enormous negative impacts on agricultural production. Animal and microbial parasites negatively affect the health of their hosts. Taken together, protists play essential roles in soil biodiversity, nutrient cycling, and agricultural productivity and provide valuable information to understand the soil ecosystem dynamics (Geisen et al. 2018). Recent studies have shown that protists may react differently from bacteria and fungi to environmental variables and are highly affected by soil-originated factors (Zhao et al. 2019; Asiloglu et al. 2021c).
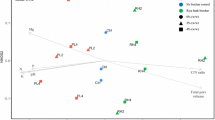
Biochar is an often-used soil amendment and one of the most important practices in sustainable agriculture (Singh et al. 2022). Biochar-induced changes in soil physicochemical properties such as increased nutrients, enhanced water holding capacity, and changes in soil pH, EC, and porosity affect the life in the soil, especially microorganisms (Palansooriya et al. 2019). In general, the effects of biochar amendment on the taxonomic composition of the microbial communities are often associated with changes in soil pH and nutrient contents, as well as the changes in physical properties of soil (Gul et al. 2015; Palansooriya et al. 2019). Depending on the source and pyrolysis method of biochar, a subset of microbial assemblage responds to specific biochar properties (Sheng and Zhu 2018; Singh et al. 2022). However, despite the mounting number of research on biochar–microbe interaction, major gaps limiting the taxonomic and functional knowledge of soil microbiome exist as the research intensity on microbial groups (viruses, bacteria, archaea, fungi, and protists) is uneven (Fig. 1). The majority of biochar research has been focused on bacteria and fungi and to a lesser extent on archaea and viruses (Fig. 1). Unquestionably, those groups are extremely important for ecosystem functioning. However, the group that plays a central role in soil functioning and constitutes the vast majority of eukaryotic diversity, protists, is mostly missing from the biochar research, representing only 0.2% of the studies on biochar–microbe interaction (Fig. 1). This article aims to present current knowledge on biochar–protist interaction, highlight the research needs and discuss potential research topics on biochar–protist interaction, which would advance our knowledge of biochar–microbe interaction.
Comparison of published biochar studies on protists with those on other microbial groups including viruses (grey), archaea (orange), bacteria (blue), and fungi (green). A) Percentage values of the total studies B) Number of published papers per years. The data represent articles available on Web of Science database. For the search crieteria and terminology of keywords, please see Geisen et al. (2017). It should be noted that the research on biochar production from protists (algae) was not included since the aim of the study is to focus on biochar-protist interaction, rather than biochar production from protists
2 Effects of biochar on protists
So far, only a few studies have focused on biochar–protist interaction (Table 1). Briefly, those studies showed that biochar alters protist community composition (Noyce et al. 2016; Asiloglu et al. 2021a), affects the population of predatory protists (Hansen et al. 2017; Liu et al. 2020), and decreases trophic interaction between bacteria and predatory protists (Liu et al. 2020; Asiloglu et al. 2021b).
Biochar is known to affect protist community composition (Noyce et al. 2016; Asiloglu et al. 2021a). Noyce et al. (2016) studied the effect of wood biochar made from sugar maple on eukaryotic communities in forest soil. Although they mainly focused on fungi, their results also included some protist groups mostly belonging to Alveolata. The relative abundance of the protists was significantly enhanced in the biochar treatment, which included flagellate and amoeba species that were exclusively present in the biochar-amended soil. A short-term in vitro study conducted in paddy field soil with two biochars originating from rice husk and poultry litter showed that the protist community composition was differently affected depending on the raw material of the biochars (Asiloglu et al. 2021a). The increased total pore volume and C/N ratio of the paddy field soil by the rice husk biochar correlated with an increase in the relative abundance of Stramenopiles. The relative abundances of Amoebozoa, Alveolata, and Excavata were increased by the poultry litter biochar, and those increases were correlated with the enhanced pH and nutrients in the soil. Although the rice husk and poultry litter biochars tended to have distinct effects on protists, the only functional group that was similarly affected (negatively) by both biochars was the plant pathogenic protists (Asiloglu et al. 2021a).
Two studies using wheat straw biochar in upland fields showed that the total population of predatory protists was enhanced by the biochar amendment (Hansen et al. 2017; Liu et al. 2020). However, by separately counting amoeba and flagellates, Liu et al (2020) showed that biochar decreases the population of amoeba, while increasing the flagellate population. In addition, Liu et al (2020) showed that biochar exerted negative impacts on the microbial trophic interactions, including predatory protists. This finding was later confirmed by Asiloglu et al. (2021b) in paddy field soil using poultry litter and rice husk biochar. The predatory effect of protists on bacterial taxa was negatively affected by both biochars depending on the applied dose. On average, the number of bacterial taxa affected by protist predation was decreased by 51.6% in 2% biochar (w/w) amended soil, while the 4% (w/w) biochar application caused 72.2% decrease in the number of bacterial taxa affected by protist predation. Overall, the results of the two studies (Liu et al. 2020; Asiloglu et al. 2021b) suggested a new insight into the effects of biochar on the soil microbiome via altering the trophic interactions.
3 Research needs
3.1 Protist diversity and community composition
Due to the limited biochar studies on protists, basic information on how biochar affects protist community composition and functionality is missing (Table 2). The knowledge obtained from bacterial and fungal studies suggests that the charosphere, the immediate soil surrounding biochar particles, provides a habitable space for microorganisms enhancing microbial activities (Palansooriya et al. 2019). However, less is known about whether a specific protist community inhabits the charosphere or not (Q1). Enhanced activities of the prey (bacteria and fungi) in the charosphere are likely to attract predatory protists; thus, the presence of predatory protists in the charosphere would not be surprising. Additionally, the charosphere that is rich in nutrients can be a potentially favorable habitat for decomposer protists such as oomycetes (Q1). Physicochemical properties of biochar such as the chemical content, pH, particle size, and pore size distribution highly depend on the raw material (Greenough et al. 2021) and the preparation methods (Amalina et al. 2022), which are crucial factors affecting the soil microbiome (Lehmann et al. 2011; Singh et al. 2022). However, still, less is known about how different raw materials (Q2), processing and preparation methods of biochar (Q3) would affect protist communities. So far, biochar research on protist community composition has been conducted in forest soil (Noyce et al. 2016) and wetland paddy field soil (Asiloglu et al. 2021a). Research on biochar–protist interaction under upland fields is needed to understand how biochar impacts protist communities under different soil types (Q4), especially in long-term field studies (Q5).
3.2 Protist functionality
Predation on bacteria and fungi is one of the major functional roles of protists (Asiloglu and Murase 2017; Gao et al. 2019; Chandarana and Amaresan 2022). Although previous studies showed that biochar amendment has a detrimental effect on multitrophic levels (Liu et al. 2020) and decreases the prey–predator interaction between protists and bacteria (Asiloglu et al. 2021b), the mechanism is not yet clarified. Although no quantitative evidence is available, it has been long hypothesized that biochar protects bacteria from predators by allowing them to explore the micro-pore habitat of itself (Lehmann et al. 2011), which is too small for predators. This hypothesis was further supported by a decreased population of relatively big-sized predators, bacterivorous nematodes and amoeba in biochar amendment soils (Kamau et al. 2019; Liu et al. 2020). On the other hand, protists can pass through channels much smaller than their body size (Wang et al. 2005) and micro-pores as small as 2 μm are accessible to many small soil flagellates and amoeba (Edwards 2003). Therefore, whether small pores of biochar provide a protective shelter to bacteria or not should be carefully tested, considering the traits of protists such as size, feeding style and most importantly their ability to access small pores (Q6). In addition, biochar is known to adsorb volatiles (Zhang et al. 2017). As microbial volatiles are directly involved in predator–prey interactions between protists and bacteria (Schulz-Bohm et al. 2017), adsorption of bacterial volatiles by biochar would potentially obscure the prey detection mechanism of some predatory protists (Q7). A simple in vitro experiment, perhaps similar to the previously established method (Schulz-Bohm et al. 2017), in the presence and absence of biochar could answer this question. Another important aspect of microbial trophic interaction is the increased microbial-driven CO2 release (Gralka et al. 2020). However, whether the decreased prey-predator interaction by biochar amendment affects soil CO2 emission or not is yet to be studied (Q8).
Not only predators but also the other functional groups of protists can be directly affected by biochar amendment. Photoautotrophic protists, especially algae, are important players in the carbon cycle, and therefore their activities are likely to be affected by biochar amendment (Q9). The role of protists in the decomposition of biochar is another important subject that should be further investigated (Q10). The plant pathogens (Q11) and animal/microbial parasites (Q12) are the other important protist functional groups that have negative impacts on the soil microbiome and agricultural productivity. Suppression of plant pathogens by biochar is well-known (Graber et al. 2014), which is also true for protists (Asiloglu et al. 2021a). Although the mechanism of how biochar suppresses protist pathogens is unknown, it is suggested that biochar-released organic compounds can be photo/biotoxic, or the volatile compounds of biochar, which have been traditionally used as pesticides could act as pathogen inhibitors for protists. Taken together, there are growing research needs on protist–biochar interaction, which is promising to extend our knowledge on biochar–microbe interaction and for further discoveries.
4 Conclusion
Despite the enormous roles of protists in soil fertility and agricultural productivity, protists are the overlooked component in biochar studies. Although protist research is often separated from bacterial and fungal studies, protists are considered a central hub in the soil ecosystem affecting the whole soil microbiome, soil fertility, and plant productivity. Therefore, the research on protist–biochar interaction would not only enhance our knowledge of protistology but also take biochar–microbe interaction to the next level. Although protist research can be challenging and requires good knowledge and skillset, the recent methodological advances in molecular biology have made studying protists more accessible to even non-experts. For instance, based on commonly used soil DNA/RNA extraction techniques, the same nucleic acid extract used to study bacteria and fungi can also be used to study protists (with a different set of PCR primers, 18s rRNA/rDNA). Therefore, the effect of biochar application on protist community composition (Q1–Q5) can be easily studied with high throughput sequencing methods. Unlike bacteria, the functional roles of protists can be estimated through DNA/RNA-based results; thus, molecular biology techniques such as high throughput sequencing create not only taxonomic but also functional knowledge of protists. This makes it possible to add protist research to even former biochar studies if nucleic acids have been properly preserved, which can accelerate research in biochar–protist interaction.
Availability of data and materials
Not applicable.
References
Amalina F, Razak ASA, Krishnan S et al (2022) Biochar production techniques utilizing biomass waste-derived materials and environmental applications—a review. J Hazard Mater Adv 7:100134. https://doi.org/10.1016/j.hazadv.2022.100134
Asiloglu R, Murase J (2017) Microhabitat segregation of heterotrophic protists in the rice (Oryza sativa L.) rhizosphere. Rhizosphere 4:82–88. https://doi.org/10.1016/j.rhisph.2017.08.001
Asiloglu R, Samuel SO, Sevilir B et al (2021a) Biochar affects taxonomic and functional community composition of protists. Biol Fertil Soils 57:15–29. https://doi.org/10.1007/s00374-020-01502-8
Asiloglu R, Sevilir B, Samuel SO et al (2021b) Effect of protists on rhizobacterial community composition and rice plant growth in a biochar amended soil. Biol Fertil Soils 57:293–304. https://doi.org/10.1007/s00374-020-01525-1
Asiloglu R, Shiroishi K, Suzuki K et al (2021c) Soil properties have more significant effects on the community composition of protists than the rhizosphere effect of rice plants in alkaline paddy field soils. Soil Biol Biochem 161:108397. https://doi.org/10.1016/j.soilbio.2021.108397
Chandarana KA, Amaresan N (2022) Soil protists: an untapped microbial resource of agriculture and environmental importance. Pedosphere 32:184–197. https://doi.org/10.1016/S1002-0160(21)60066-8
Edwards CA (2003) Encyclopedia of soil science. Appl Soil Ecol 23:279. https://doi.org/10.1016/S0929-1393(03)00051-9
Gao Z, Karlsson I, Geisen S et al (2019) Protists: puppet masters of the rhizosphere microbiome. Trends Plant Sci 24:165–176. https://doi.org/10.1016/j.tplants.2018.10.011
Geisen S, Mitchell EAD, Wilkinson DM et al (2017) Soil protistology rebooted: 30 fundamental questions to start with. Soil Biol Biochem 111:94–103. https://doi.org/10.1016/j.soilbio.2017.04.001
Geisen S, Mitchell EAD, Adl S et al (2018) Soil protists: a fertile frontier in soil biology research. FEMS Microbiol Rev 42:293–323. https://doi.org/10.1093/femsre/fuy006
Graber ER, Frenkel O, Jaiswal AK, Elad Y (2014) How may biochar influence severity of diseases caused by soilborne pathogens? Carbon Manag 5:169–183. https://doi.org/10.1080/17583004.2014.913360
Gralka M, Szabo R, Stocker R, Cordero OX (2020) Trophic interactions and the drivers of microbial community assembly. Curr Biol 30:R1176–R1188. https://doi.org/10.1016/j.cub.2020.08.007
Greenough S, Dumont M-J, Prasher S (2021) The physicochemical properties of biochar and its applicability as a filler in rubber composites: a review. Mater Today Commun 29:102912. https://doi.org/10.1016/j.mtcomm.2021.102912
Gul S, Whalen JK, Thomas BW et al (2015) Physico-chemical properties and microbial responses in biochar-amended soils: mechanisms and future directions. Agric Ecosyst Environ 206:46–59. https://doi.org/10.1016/j.agee.2015.03.015
Guo S, Xiong W, Hang X et al (2021) Protists as main indicators and determinants of plant performance. Microbiome 9:64. https://doi.org/10.1186/s40168-021-01025-w
Hansen V, Müller-Stöver D, Imparato V et al (2017) The effects of straw or straw-derived gasification biochar applications on soil quality and crop productivity: a farm case study. J Environ Manage 186:88–95. https://doi.org/10.1016/j.jenvman.2016.10.041
Jassey VEJ, Walcker R, Kardol P et al (2022) Contribution of soil algae to the global carbon cycle. New Phytol 234:64–76. https://doi.org/10.1111/nph.17950
Kamau S, Karanja NK, Ayuke FO, Lehmann J (2019) Short-term influence of biochar and fertilizer-biochar blends on soil nutrients, fauna and maize growth. Biol Fertil Soils 55:661–673. https://doi.org/10.1007/s00374-019-01381-8
Lehmann J, Rillig MC, Thies J et al (2011) Biochar effects on soil biota—a review. Soil Biol Biochem 43:1812–1836. https://doi.org/10.1016/j.soilbio.2011.04.022
Liu T, Yang L, Hu Z et al (2020) Biochar exerts negative effects on soil fauna across multiple trophic levels in a cultivated acidic soil. Biol Fertil Soils. https://doi.org/10.1007/s00374-020-01436-1
Noyce GL, Winsborough C, Fulthorpe R, Basiliko N (2016) The microbiomes and metagenomes of forest biochars. Sci Rep 6:26425. https://doi.org/10.1038/srep26425
Palansooriya KN, Wong JTF, Hashimoto Y et al (2019) Response of microbial communities to biochar-amended soils: a critical review. Biochar 1:3–22. https://doi.org/10.1007/s42773-019-00009-2
Schulz-Bohm K, Geisen S, Wubs ERJ et al (2017) The prey’s scent—volatile organic compound mediated interactions between soil bacteria and their protist predators. ISME J 11:817–820. https://doi.org/10.1038/ismej.2016.144
Sheng Y, Zhu L (2018) Biochar alters microbial community and carbon sequestration potential across different soil pH. Sci Total Environ 622–623:1391–1399. https://doi.org/10.1016/j.scitotenv.2017.11.337
Singh H, Northup BK, Rice CW, Prasad PVV (2022) Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: a meta-analysis. Biochar 4:8. https://doi.org/10.1007/s42773-022-00138-1
Wang W, Shor LM, LeBoeuf EJ et al (2005) Mobility of protozoa through narrow channels. Appl Environ Microbiol 71:4628–4637. https://doi.org/10.1128/AEM.71.8.4628-4637.2005
Xiong W, Song Y, Yang K et al (2020) Rhizosphere protists are key determinants of plant health. Microbiome 8:1–9. https://doi.org/10.1186/s40168-020-00799-9
Zhang X, Gao B, Zheng Y et al (2017) Biochar for volatile organic compound (VOC) removal: sorption performance and governing mechanisms. Bioresour Technol 245:606–614. https://doi.org/10.1016/j.biortech.2017.09.025
Zhao Z-B, He J-Z, Geisen S et al (2019) Protist communities are more sensitive to nitrogen fertilization than other microorganisms in diverse agricultural soils. Microbiome 7:33. https://doi.org/10.1186/s40168-019-0647-0
Acknowledgements
I would like to thank to anonymous reviewers who made valuable comments which helped to improve the manuscript.
Funding
This research was funded by the Japan Society for the Promotion of Science (JSPS) to Asiloglu R (Grant No. JP22K14804).
Author information
Authors and Affiliations
Contributions
Not applicable (Single author manuscript).
Corresponding author
Ethics declarations
Competing interests
The author has no relevant financial or non-financial interests to disclose.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Asiloglu, R. Biochar–microbe interaction: more protist research is needed. Biochar 4, 72 (2022). https://doi.org/10.1007/s42773-022-00195-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-022-00195-6