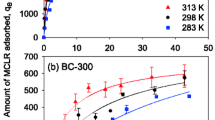
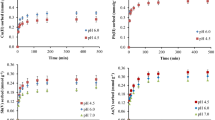
Abstract
In this study, we report on the extraction, characterization, and potential applications of colloidal biochar derived from pyrolyzed wood—an untapped source of carbonaceous particles. A series of characterizations was performed on biochar colloids to unravel their colloidal properties and surface chemistry through which it was found that they have a net negative charge and are stable between pH 3 and 10. Moreover, our initial toxicity tests showed that biochar colloids themselves are not toxic and they can be used in remediation applications, which led us to investigate (1) their copper sorption, a model inorganic contaminant, in a scenario that biochar colloids are released into the environment and (2) their potential use in organic pollutants adsorption and degradation. Copper sorption studies showed that biochar colloids have a copper sorption capacity as high as 22 mg \(\hbox {g}^{-1}\) in sub-ppm copper solutions. This increased the acute 48 h lethal concentration (\(\hbox {LC}_{50}\)) of copper for Daphnia magna by 21 ppb, which is comparable to the previously reported effect by dissolved organic matter. Adsorption and degradation of methylene blue (MB), an often-used proxy for organic contaminants in water, were studied by coupling the biochar colloids to positively charged \(\hbox {TiO}_{2}\) nanoparticles and using it as a photocatalyst. The hybrid MB photodegradation efficiency was \(21\%\) higher than that of \(\hbox {TiO}_{2}\) nanoparticles alone. Enhancement of demethylation is proposed as the main degradation mechanism of MB, as confirmed by liquid chromatography–mass spectroscopy (LC/MS), and the positive impact of biochar colloids is ascribed to their abundant adsorption sites, which may facilitate MB adsorption and its photocatalytic degradation.













Similar content being viewed by others
References
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33
Alam MS, Gorman-Lewis D, Chen N, Flynn SL, Ok YS, Konhauser KO, Alessi DS (2018a) Thermodynamic analysis of nickel(ii) and zinc(ii) adsorption to biochar. Environ Sci Technol 52(11):6246–6255
Alam MS, Swaren L, von Gunten K, Cossio M, Bishop B, Robbins LJ, Hou D, Flynn SL, Ok YS, Konhauser KO, Alessi DS (2018b) Application of surface complexation modeling to trace metals uptake by biochar-amended agricultural soils. Appl Geochem 88(Part A):103–112
Albanese KA, Lanno RP, Hadad CM, Chin Y-P (2017) Photolysis- and dissolved organic matter-induced toxicity of triclocarban to daphnia magna. Environ Sci Technol Lett 4:457–462
Blewett TA, Dow EM, Wood CM, McGeer JC, Smith DS (2018) The role of dissolved organic carbon concentration and composition on nickel toxicity to early life-stages of the blue mussel mytilus edulis and purple sea urchin strongylocentrotus purpuratus. Ecotoxico. Environ Saf 160:162–170
Boudart M (1956) Kinetics on ideal and real surfaces. A I Ch E J 1:62–64
Cai X, Li J, Liu Y, Yan Z, Tan X, Liu S, Zeng G, Gu Y, Hu X, Jiang L (2017) Titanium dioxide-coated biochar composites as adsorptive and photocatalytic degradation materials for the removal of aqueous organic pollutants. ACS Sustain Chem Eng 93(3):783–791
Cao Y, Xiao W, Shen G, Ji G, Zhang Y, Gao C, Han L (2019) Carbonization and ball milling on the enhancement of pb(ii) adsorption by wheat straw: Competitive effects of ion exchange and precipitation. Bioresour Technol 273:70–76
Chen M, Wang D, Yang F, Xu X, Xu N, Cao X (2017) Transport and retention of biochar nanoparticles in a paddy soil under environmentally-relevant solution chemistry conditions. Environ Pollut 230:540–549
Chen M, Wang D, Yang F, Xu X, Xu N, Cao X (2018) Contrasting effects of biochar nanoparticles on the retention and transport of phosphorus in acidic and alkaline soils. Environ Pollut 239:562–570
Chen X, Chen G, Chen L, Chen Y, Lehmann J, McBride MB, Hay AG (2011) Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresour Technol 102(19):8877–8884
Colmenares JC, Varma RS, Lisowski P (2016) Sustainable hybrid photocatalysts: titania immobilized on carbon materials derived from renewable and biodegradable resources. Green Chem 18:5736–5750
Colthup NB, Daly LH, Wiberley SE (1990) Introduction to infrared and raman spectroscopy (third edition). Academic Press, Cambridge
Dong X, He L, Hu H, Liu N, Gao S, Piao Y (2018) Removal of \(17\beta\)-estradiol by using highly adsorptive magnetic biochar nanoparticles from aqueous solution. Chem Eng J 352:371–379
Fang G, Liu C, Wang Y, Dionysiou DD, Zhou D (2017) Photogeneration of reactive oxygen species from biochar suspension for diethyl phthalate degradation. Appl Catal B 214:34–45
Ferrari AC, Robertson J (2000) Interpretation of raman spectra of disordered and amorphous carbon. Phys Rev B 61(20):14095–14107
Fu H, Liu H, Mao J, Chu W, Li Q, Alvarez PJJ, Qu X, Zhu D (2016) Photochemistry of dissolved black carbon released from biochar: Reactive oxygen species generation and phototransformation. Environ Sci Technol 50:1218–1226
Ghaffar A, Zhu X, Chen B (2018) Biochar composite membrane for high performance pollutant management: Fabrication, structural characteristics and synergistic mechanisms. Environ Pollut 233:1013–1023
He P, Yu Q, Zhang H, Shao L, Lu F (2018) Removal of copper (ii) by biochar mediated by dissolved organic matter. Sci Rep 7:7091
Ho Y-S, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465
Ho Y-S, Porter JF, McKay G (2002) Equilibrium isotherm studies for the sorption of divalent metal ions onto peat: copper, nickel and lead single component systems. Water Air Soil Pollut 141(1):1–33
Hunter RJ (1981) Zeta potential in colloid science. Academic Press, Cambridge
Leary R, Westwood A (2011) Carbonaceous nanomaterials for the enhancement of \(\text{TiO}_{2}\) photocatalyis. Carbon 49(3):741–772
Li L, Zhang K, Chen L, Huang Z, Liu G, Li M, Wen Y (2017a) Mass preparation of micro/nano-powders of biochar with water-dispersibility and their potential application. New J Chem 41:9649–9657
Li M, Liu Q, Lou Z, Wang Y, Zhang Y, Qian G (2014) Method to characterize acid-base behavior of biochar: site modeling and theoretical simulation. ACS Sustain Chem Eng 2(11):2501–2509
Li M, Zhang A, Wua H, Liu H, Lv J (2017b) Predicting potential release of dissolved organic matter from biocharsderived from agricultural residues using fluorescence and ultravioletabsorbance. J Hazard Mater 334:86–92
Li X, Yu J, Wageh S, Al-Ghamdi AA, Xie J (2016) Graphene in photocatalysis: a review. Small 12(48):6640–6696
Li Y, Sun S, Ma M, Ouyang Y, WenbinYan (2008) Kinetic study and model of the photocatalytic degradation of rhodamine b (rhb) by a \(\text{TiO}_{2}\)-coated activated carbon catalyst: effects of initial rhb content, light intensity and \(\text{TiO}_{2}\) content in the catalyst. Chem Eng J 142(2):147–155
Lin C, Song Y, Cao L, Chen S (2013) Effective photocatalysis of functional nanocomposites based on carbon and \(\text{TiO}_2\) nanoparticles. Nanoscale 5(11):4986–4992
Lisowski P, Colmenares JC, Masek O, Lisowski W, Lisovytskiy D, Kaminska A, Lomot D (2017) Dual functionality of \(\text{TiO}_{2}\)/biochar hybrid materials: photocatalytic phenol degradation in the liquid phase and selective oxidation of methanol in the gas phase. ACS Sustain Chem Eng 5(7):6274–6287
Liu G, Zheng H, Jiang Z, Zhao J, Wang Z, Pan B, Xing B (2018) Formation and physicochemical characteristics of nano biochar: Insight into chemical and colloidal stability. Environ Sci Technol 52:10369–10379
Lucchese MM, Stavale F, Ferreira EHM, Vilani C, Moutinho MVO, B.Capaz R, Achete CA, Jorio A (2010) Quantifying ion-induced defects and raman relaxation length in graphene. Carbon 48(5):1592–1597
Lyu H, Gao B, Hed F, Zimmerman AR, Ding C, Tang J, Crittenden CJ (2018) Experimental and modeling investigations of ball-milled biochar for the removal of aqueous methylene blue. Chem Eng J 335:110–119
Manya JJ (2012) Pyrolysis for biochar purposes: a review to establish current knowledge gaps and research needs. Environ Sci Technol 46(15):7939–7954
Mao C-C, Weng H-S (2009) Promoting effect of adding carbon black to tio2 for aqueous photocatalytic degradation of methyl orange. Chem Eng J 155(3):744–749
Mattevi C, Eda G, Agnoli S, Miller S, Mkhoyan KA, Celik O, Mastrogiovanni D, Granozzi G, Garfunkel E, Chhowa M (2009) Evolution of electrical, chemical, and structural properties of transparent and conducting chemically derived graphene thin films. Adv Funct Mater 19:2577–2583
Milan MM, Liu G (2019) Sewage sludge-derived \(\text{TiO}_{2}/\text{Fe}/\text{Fe}_{3}\text{C}\)-biochar composite as an efficient heterogeneous catalyst for degradation of methylene blue. Chemosphere 215:101–114
Naghdi M, Taheran M, Brar SK, Rouissia T, Verma M, Surampalli RY, Valero JR (2017) A green method for production of nanobiochar by ball milling-optimization and characterization. J Clean Prod 164:1394–1405
OECD (2008) OECD. Guideline for testing of chemicals; No. 211, Daphnia magna reproduction test. OECD
Oleszczuk P, Cwikla-Bundyra W, Bogusz A, Skwarek E, Ok YS (2016) Characterization of nanoparticles of biochars from different biomass. J Anal Appl Pyrolysis 121:165–172
Oliveira FR, Patel AK, Jaisi PD, Adhikari S, Lud H, Khanal SK (2017) Environmental application of biochar: current status and perspectives. Bioresour Technol 246:110–122
Qian L, Zhang W, Yan J, Han L, Gao W, Liu R, Chen M (2016) Effective removal of heavy metal by biochar colloids under different pyrolysis temperatures. Bioresour Technol 206:217–224
Qu X, Fu H, Mao J, Ran Y, Zhang D, Zhu D (2016) Chemical and structural properties of dissolved black carbon released from biochars. Carbon 96:759–767
Rauf MA, Meetani MA, Khaleel A, Ahmed A (2010) Photocatalytic degradation of methylene blue using a mixed catalyst and product analysis by lc/ms. Chem Eng J 157:373–378
Schamphelaere KACD, Vasconcelos FM, Tack FMG, Allen HE, Janssen CR (2004) Effect of dissolved organic matter source on acute copper toxicity to daphnia magna. Environ Toxicol Chem 23(5):1248–1255
Sevilla M, Fuertes AB (2009) The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 47(9):2281–2289
Song B, Chen M, Zhao L, Qiu H, Cao X (2019) Physicochemical property and colloidal stability of micron- and nano-particle biochar derived from a variety of feedstock sources. Sci Total Environ 661:685–695
Sun Y, Wu Z-Y, Wang X, Ding C, Cheng W, Yu S-H, Wang X (2016) Macroscopic and microscopic investigation of u(vi) and eu(iii) adsorption on carbonaceous nanofibers. Environ Sci Technol 50(8):4459–4467
Tan X, Guo LY, Ling GY, Xu Y, Ming ZG, Jiang HX, Bo LS, Wang X, Mian LS, Li J (2016) Biochar-based nano-composites for the decontamination of wastewater: a review. Bioresour Technol 212:318–333
Tang J, Li X, Luo Y, Li G, Khan S (2016) Spectroscopic characterization of dissolved organic matter derived from different biochars and their polycylic aromatic hydrocarbons (pahs) binding affinity. Chemosphere 152:399–406
Ursachi I, Stancu A, Vasile A (2012) Magnetic \(\alpha\)-\(\text{Fe}_{2}\text{O}_{3}/\text{mcm}\)-\(41\) nanocomposites: preparation, characterization, and catalytic activity for methylene blue degradation. J Colloid Interface Sci 377(1):184–190
von Gunten K, Alam MS, Hubmann M, Ok YS, Konhauser KO, Alessi DS (2017) Modified sequential extraction for biochar and petroleum coke: metal release potential and its environmental implications. Bioresour Technol 236:106–110
Wang B, Gao B, Wan Y (2018) Entrapment of ball-milled biochar in ca-alginate beads for the removal of aqueous cd(ii). J Ind Eng Chem 61:161–168
Wang D, Zhang W, Hao X, Zhou D (2013a) Transport of biochar particles in saturated granular media: Effects of pyrolysis temperature and particle size. Environ Sci Technol 47:821–828
Wang D, Zhang W, Zhou D (2013b) Antagonistic effects of humic acid and iron oxyhydroxide grain coating on biochar nanoparticle transport in saturated sand. Environ Sci Technol 47(10):5154–5161
Xiao X, Chen B, Chen Z, Zhu L, Schnoor JL (2018) Insight into multiple and multilevel structures of biochars and their potential environmental applications: A critical review. Environ Sci Technol 52(9):5027–5047
Xie T, Reddy KR, Wang C, Yargicoglu E, Spokas K (2015) Characteristics and applications of biochar for environmental remediation: a review. Crit Rev Env Sci Tech 45:939–969
Xu C, Rangaiah GP, Zhao XS (2014) Photocatalytic degradation of methylene blue by titanium dioxide: experimental and modeling study. Ind Eng Chem Res 53(38):14641–14649
Yang W, Shang J, Sharma P, Li B, Liu K, Flury M (2019) Colloidal stability and aggregation kinetics of biochar colloids: effects of pyrolysis temperature, cation type, and humic acid concentrations. Sci Total Environ 658:1306–1315
Yang Z-C, Li X, Wang J (2011) Intrinsically fluorescent nitrogen-containing carbon nanoparticles synthesized by a hydrothermal process. Carbon 49(49):5207–5212
Yi P, Pignatello JJ, Uchimiya M, White JC (2015) Heteroaggregation of cerium oxide nanoparticles and nanoparticles of pyrolyzed biomass. Environ Sci Technol 49(22):13294–13303
Yue L, Liana F, Han Y, Bao Q, Wang Z, Xing B (2019) The effect of biochar nanoparticles on rice plant growth and the uptake of heavy metals: implications for agronomic benefits and potential risk. Sci Total Environ 656:9–18
Zhang C, Lohwacharin J, Takizawa S (2017) Properties of residual titanium dioxide nanoparticles after extended periods of mixing and settling in synthetic and natural waters. Sci Rep 7:9943
Zhang N, Zhang Y, Xu Y-J (2012) Recent progress on graphene-based photocatalysts: current status and future perspectives. Nanoscale 4:5792–5813
Zhang T, Oyama T, Aoshima A, Hidaka H, Zhao J, Serpone N (2001) Photooxidative n-demethylation of methylene blue in aqueous \(\text{TiO}_{2}\) dispersions under uv irradiation. J Photochem Photobiol A 140(2):163–172
Zhang Y, Tang Z-R, Fu X, Xu Y-J (2010) \(\text{TiO}_{2}\)-graphene nanocomposites for gas-phase photocatalytic degradation of volatile aromatic pollutant: Is \(\text{TiO}_{2}\)-graphene truly different from other \(\text{TiO}_{2}\)-carbon composite materials? ACS Nano 4(12):7303–7314
Zhou Z, Chen B, Qu X, Fu H, Zhu D (2018) Dissolved black carbon as an efficient sensitizer in the photochemical transformation of \(17\alpha\)-estradiol in aqueous solution. Environ Sci Technol 52:10391–10399
Acknowledgements
This work was supported by a Natural Sciences and Engineering Research Council (NSERC) Discovery grant (RGPIN-04134) to D.S.A. The authors would like to thank Prof. Jonathan Curtis in department of Agricultural, Food, and Nutritional Science and Prof. Al Meldrum in the Department of Physics at the University of Alberta for the use of FTIR and photoluminescence spectroscopy, respectively. Furthermore, the authors are grateful to Prof. Jonathan G. C. Veinot and Maryam Aghajamali in the Department of Chemistry at the University of Alberta for the use of a Zetasizer.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Safari, S., von Gunten, K., Alam, M.S. et al. Biochar colloids and their use in contaminants removal. Biochar 1, 151–162 (2019). https://doi.org/10.1007/s42773-019-00014-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42773-019-00014-5