Abstract
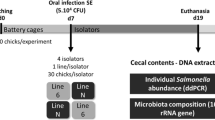
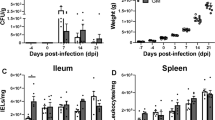
Slow-growing breeds are more resistant to Salmonella infection compared to fast-growing broilers. However, it is unclear whether that is associated with innate resistance or rather rely on differences in Salmonella-induced gut responses. We investigated the microbial composition and gene expression of nutrient transporters, mucin, and interleukin in the gut of a fast-growing (Cobb500) and a slow-growing naked neck (NN) chicken breeds challenged with Salmonella Enteritidis. Hatchlings were inoculated at two days of age using sterile broth (sham) or Salmonella Enteritidis (SE) and distributed according to a completely randomized design into four treatments: Cobb-sham; Cobb-SE; NN-sham; and NN-SE. Cecal SE counting and microbial composition by 16 S rRNA sequencing were determined at 24-, 96-, and 168-hours post-inoculation (hpi). Gene expression of amino acid (Asct1) and peptide transporters (PepT1), glucose transporters (Sglt1, Glut2 and Glut5) and mucin (Muc2) in the jejunum and expression of interleukins (IL1 beta, IL8, IL17 and IL22) in the cecum was assessed by qPCR at 24 and 168 hpi. NN birds were colonized by SE just as Cobb birds but showed innate upregulation of Muc2, IL8 and IL17 in comparison to Cobb. While nutrient transporter mRNA expression was impaired in SE-challenged Cobb birds, the opposite was observed in NN. There were no differences in microbial diversity at different sampling times for Cobb-SE, whereas the other groups had higher diversity and lower dominance at 24 hpi compared with 96 hpi and 168 hpi. NN birds apparently develop earlier gut microbial stability, have higher basal level of mucin gene expression as well as differential nutrient transporter and interleukin gene expression in the presence of SE which might mitigate the effects of SE infection compared to Cobb birds.



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available but can be made available from the corresponding author on reasonable request.
References
Bröer S (2008) Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 88:249–286. https://doi.org/10.1152/physrev.00018.2006
Barri A, Honaker CF, Sottosanti JR, Hulet RM, McElroy AP (2011) Effect of incubation temperature on nutrient transporters and small intestine morphology of broiler chickens. Poult Sci 90:118–125. https://doi.org/10.3382/ps.2010-00908
Miska KB, Fetterer RH (2019) Expression of amino acid and sugar transporters, aminopeptidase, and the di- and tri-peptide transporter PepT1; differences between modern fast growing broilers and broilers not selected for rapid growth. Poult Sci 98:2272–2280. https://doi.org/10.3382/ps/pey583
Zhang H, Li D, Liu L, Xu L, Zhu M, He X, Liu Y (2019) Cellular composition and differentiation signaling in chicken small intestinal epithelium. Anim (Basel) 11:870. https://doi.org/10.3390/ani9110870
Gilbert ER, Li H, Emmerson DA, Webb KE Jr, Wong EA (2007) Developmental regulation of nutrient transporter and enzyme mRNA abundance in the small intestine of broilers. Poult Sci 86:1739–1753. https://doi.org/10.1093/ps/86.8.1739
Gunal M, Yayli G, Kaya O, Karahan N, Sulak O (2006) The effect of antibiotic growth promoter, probiotic or organic acid supplementation on performance, intestinal microflora and tissue of broilers. Int J Poult Sci 5:149–155. https://doi.org/10.3923/ijps.2006.149.155
Rychlik I, Elsheimer-Matulova M, Kyrova K (2014) Gene expression in the chicken caecum in response to infections with non-typhoid Salmonella. Vet Res 45:119. https://doi.org/10.1186/s13567-014-0119-2
ECDC EFSA (2017) Multicountry outbreak of Salmonella Enteritidis infections linked to Polish eggs. European Centre for Disease Prevention and Control and European Food Safety Authority: Stockholm and Parma. http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1353/pdf. Accessed Apr 2020
Van Immerseel F, De Buck J, De Smet I, Mast J, Haesebrouck F, Ducatelle R (2002) The effect of vaccination with a Salmonella Enteritidis aroA mutant on early cellular responses in caecal lamina propria of newly-hatched chickens. Vaccine 20:3034–3041. https://doi.org/10.1016/s0264-410x(02)00227-x
Bayyari GR, Huff WE, Rath NC, Balog JM, Newberry LA, Villines JD, Skeeles JK, Anthony NB, Nestor KE (1997) Effect of the genetic selection of turkeys for increased body weight and egg production on immune and physiological responses. Poult Sci 76:289–296. https://doi.org/10.1093/ps/76.2.289
Crossley DA II, Altimiras J (2012) Effect of selection for commercially productive traits on the plasticity of cardiovascular regulation in chicken breeds during embryonic development. Poult Sci 91:2628–2636. https://doi.org/10.3382/ps.2012-02344
Wigley P (2004) Genetic resistance to Salmonella infection in domestic animals. Res Vet Sci 76:165–169. https://doi.org/10.1016/S0034-5288(03)00117-6
Coble DJ, Redmond SB, Hale B, Lamont SJ (2011) Distinct lines of chickens express different splenic cytokine profiles in response to Salmonella Enteritidis challenge. Poult Sci 90:1659–1663. https://doi.org/10.3382/ps.2010-01279
Redmond SB, Chuammitri P, Andreasen CB, Palić D, Lamont SJ (2009) Chicken heterophils from commercially selected and non-selected genetic lines express cytokines differently after in vitro exposure to Salmonella enteritidis. Vet Immunol Immunopathol 132:129–134. https://doi.org/10.1016/j.vetimm.2009.05.010
Van Hemert S, Hoekman AJ, Smits MA, Rebel JM (2006) Gene expression responses to a Salmonella infection in the chicken intestine differ between lines. Vet Immunol Immunopathol 114:247–258. https://doi.org/10.1016/j.vetimm.2006.08.007
Cheng HW, Eicher SD, Chen Y, Singleton P, Muirt WM (2001) Effect of genetic selection for group productivity and longevity on immunological and hematological parameters of chickens. Poult Sci 80:1079–1086. https://doi.org/10.1093/ps/80.8.1079
Davison F, Kaspers B, Schat KA (2008) Avian immunology. Elsevier, Amsterdam
Rostagno HS, Albino LFT, Donzele JL, Gomes PC, Oliveira RF, Lopes DC, Ferreira AS, Barreto SLT, Euclides RF (2011) Tabelas brasileiras para aves e suínos: composição de alimentos e exigências nutricionais, 3rd edn. Universidade Federal de Viçosa, Viçosa
Moreira Filho ALB, Oliveira CJB, Freitas Neto OC, de Leon C, Saraiva M, Andrade M, White B, Givisiez PEN (2018) Intra-amnionic threonine administered to chicken embryos reduces Salmonella enteritidis cecal counts and improves posthatch intestinal development. J Immunol Res. https://doi.org/10.1155/2018/9795829
Uni Z, Geyra A, Ben-Hur H, Sklan D (2000) Small intestinal development in the young chick: crypt formation and enterocyte proliferation and migration. Br Poult Sci 41:544–551. https://doi.org/10.1080/00071660020009054
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Caporaso JG (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. https://doi.org/10.1038/s41587-019-0209-9
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/. Accessed Apr 2020
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods (San DiegoCalif) 25:402–408. https://doi.org/10.1006/meth.2001.1262
Chang CH, Teng PY, Lee TT, Yu B (2020) Effects of multi-strain probiotic supplementation on intestinal microbiota, tight junctions, and inflammation in young broiler chickens challenged with Salmonella enterica subsp. Enterica. Asian-Australas J Anim Sci 33:1797–1808. https://doi.org/10.5713/ajas.19.0427
Ozaydin T, Celik I (2012) Histological, histochemical and immunohistochemical investigations on the developing small intestines of broilers embryos. J Anim Vet Adv 11:2936–2944. https://doi.org/10.3923/javaa.2012.2936.2944
Reynolds KL, Cloft SE, Wong EA (2020) Changes with age in density of goblet cells in the small intestine of broiler chicks. Poult Sci 99:2342–2348. https://doi.org/10.1016/j.psj.2019.12.052
Forder RE, Nattrass GS, Geier MS, Hughes RJ, Hynd PI (2012) Quantitative analyses of genes associated with mucin synthesis of broiler chickens with induced necrotic enteritis. Poult Sci 91:1335–1341. https://doi.org/10.3382/ps.2011-02062
Golder HM, Geier MS, Forder RE, Hynd PI, Hughes RJ (2011) Effects of necrotic enteritis challenge on intestinal micro-architecture and mucin profile. Br Poult Sci 52:500–506. https://doi.org/10.1080/00071668.2011.587183
Duarte CR, Vicentini-Paulino ML, Buratini J Jr, Castilho AC, Pinheiro DF (2011) Messenger ribonucleic acid abundance of intestinal enzymes and transporters in feed-restricted and refed chickens at different ages. Poult Sci 90:863–868. https://doi.org/10.3382/ps.2010-01015
Ebrahimi R, Faseleh Jahromi M, Liang JB, Soleimani Farjam A, Shokryazdan P, Idrus Z (2015) Effect of dietary lead on intestinal nutrient transporters mRNA expression in broiler chickens. Biomed Res Int. https://doi.org/10.1155/2015/149745
Ruhnke I, Röhe I, Goodarzi Boroojeni F, Knorr F, Mader A, Hafeez A, Zentek J (2015) Feed supplemented with organic acids does not affect starch digestibility, nor intestinal absorptive or secretory function in broiler chickens. J Anim Physiol Anim Nutr (Berl) 99(Suppl S1):29–35. https://doi.org/10.1111/jpn.12313
Criado-Mesas L, Abdelli N, Noce A, Farré M, Pérez JF, Solà-Oriol D, Martin-Venegas R, Forouzandeh A, González-Solé F, Folch JM (2021) Transversal gene expression panel to evaluate intestinal health in broiler chickens in different challenging conditions. Sci Rep 11:6315. https://doi.org/10.1038/s41598-021-85872-5
Mowat AM (2003) Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol 3:331–341. https://doi.org/10.1038/nri1057
Beal RK, Powers C, Davison TF, Smith AL (2006) Immunological development of the avian gut. In: Perry GC (ed) Avian gut function in Health and Disease. CAB International, Wallingford, pp 85–103
Kogut MH, Swaggerty CL, Byrd JA, Selvaraj R, Arsenault RJ (2016) Chicken-specific kinome array reveals that Salmonella enterica serovar Enteritidis modulates host immune signaling pathways in the cecum to establish a persistence infection. Int J Mol Sci 17:1207. https://doi.org/10.3390/ijms17081207
Bar-Shira E, Friedman A (2006) Development and adaptations of innate immunity in the gastrointestinal tract of the newly hatched chick. Dev Comp Immunol 30:930–941. https://doi.org/10.1016/j.dci.2005.12.002
Kaiser P, Rothwell L, Galyov EE, Barrow PA, Burnside J, Wigley P (2000) Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis and Salmonella gallinarum. Microbiology 146:3217–3226. https://doi.org/10.1099/00221287-146-12-3217
Crhanova M, Hradecka H, Faldynova M, Matulova M, Havlickova H, Sisak F, Rychlik I (2011) Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar enteritidis infection. Infect Immun 79:2755–2763. https://doi.org/10.1128/IAI.01375-10
Reynolds JM, Angkasekwinai P, Dong C (2010) IL-17 family member cytokines: regulation, and function in innate immunity. Cytokine Growth Factor Rev 21:413–423. https://doi.org/10.1016/j.cytogfr.2010.10.002
Rutz S, Eidenschenk C, Ouyang W (2013) IL-22, not simply a Th17 cytokine. Immunol Rev 252:116–132. https://doi.org/10.1111/imr.12027
Pan D, Yu Z (2014) Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 5:108–119. https://doi.org/10.4161/gmic.26945
Kosiewicz MM, Zirnheld AL, Alard P (2011) Gut microbiota, immunity, and disease: a complex relationship. Front Microbiol 2:180. https://doi.org/10.3389/fmicb.2011.00180
Videnska P, Sisak F, Havlickova H, Faldynova M, Rychlik I (2013) Influence of Salmonella enterica Serovar Enteritidis infection on the composition of chicken cecal microbiota. BMC Vet Res 9:1–8. https://doi.org/10.1186/1746-6148-9-140
Liu G, Zhu H, Ma T, Yan Z, Zhang Y, Geng Y, Zhu Y, Shi Y (2020) Effect of chronic cyclic heat stress on the intestinal morphology, oxidative status and cecal bacterial communities in broilers. J Therm Biol 91:102619. https://doi.org/10.1016/j.jtherbio.2020.102619
Pedroso AA, Lee MD, Maurer JJ (2021) Strength lies in diversity: how community diversity limits salmonella abundance in the chicken intestine. Front Microbiol 12:694215. https://doi.org/10.3389/fmicb.2021.694215
Acknowledgements
The authors thank to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Finance code 001) for scholarships granted to MRBS, ALBMF, MFSA, NVMS, GFCS and MLPL; Conselho Nacional de Pesquisa e Desenvolvimento (CNPq) for scholarship (311860/2016-8) and research funding granted to PENG (458340/2014-6) and Financiadora de Estudos e Projetos (FINEP) for equipment supply in the scope of MCT-Infra institutional projects.
Author information
Authors and Affiliations
Contributions
ALBMF, PENG, MFSA and CJBO conceived and designed research. MRBS, MFSA, NMVS, MLPL, GFCS conducted experiment, laboratorial and bioinformatic analyses. CJBO and OCFN contributed with reagents. MRBS, PENG, ALBMF, OCFN, MFSA, NMVS, GS, MLPL and CJBO analyzed data. MRBS, PENG, CJBO and GFCS wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All management practices, as well as slaughter and sampling procedures were approved ty the Ethical Committee for the use of Animals from Universidade Federal da Paraiba (protocol 186/15) in compliance with the National Council for Animal Experimentation Control – CONCEA [Conselho Nacional de Controle de Experimentação Animal - CONCEA] (Federal Law nº 11.794/08, Lei Arouca) as established in art. 225 of the Brazilian National Constitution on the guidance for the use of animals for scientific purposes.
Competing interests
The authors state that there is no conflict of interest.
Additional information
Responsible Editor: David Germano Gonçalves Schwarz
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1.26 MB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santos, M.R.B., Moreira Filho, A.L., Freitas Neto, O.C. et al. Shifts in microbiota and gene expression of nutrient transporters, mucin and interleukins in the gut of fast-growing and slow-growing chickens infected by Salmonella Enteritidis. Braz J Microbiol (2024). https://doi.org/10.1007/s42770-024-01297-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42770-024-01297-y