Abstract
N-Acetyl-glucosaminidases (GlcNAcases) are exoenzymes found in a wide range of living organisms, which have gained great attention in the treatment of disorders related to diabetes, Alzheimer’s, Tay-Sachs’, and Sandhoff’s diseases; the control of phytopathogens; and the synthesis of bioactive GlcNAc-containing products. Aiming at future industrial applications, in this study, GlcNAcase production by marine Aeromonas caviae CHZ306 was enhanced first in shake flasks in terms of medium composition and then in bench-scale stirred-tank bioreactor in terms of physicochemical conditions. Stoichiometric balance between the bioavailability of carbon and nitrogen in the formulated culture medium, as well as the use of additional carbon and nitrogen sources, played a central role in improving the bioprocess, considerably increasing the enzyme productivity. The optimal cultivation medium was composed of colloidal α-chitin, corn steep liquor, peptone A, and mineral salts, in a 5.2 C:N ratio. Optimization of pH, temperature, colloidal α-chitin concentration, and kLa conditions further increased GlcNAcase productivity. Under optimized conditions in bioreactor (i.e., 34 °C, pH 8 and kLa 55.2 h−1), GlcNAcase activity achieved 173.4 U.L−1 after 12 h of cultivation, and productivity no less than 14.45 U.L−1.h−1 corresponding to a 370-fold enhancement compared to basal conditions.
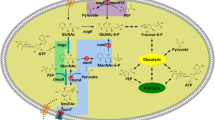
Graphical Abstract




Similar content being viewed by others
References
Slámová K, Bojarová P, Petrásková L, Křen V (2010) β-N-Acetylhexosaminidase: what’s in a name…? Biotechnol Adv 28(6):682–693. https://doi.org/10.1016/J.BIOTECHADV.2010.04.004
Hyskova V, Ryšlavá H (2015) Different roles of β-N-Acetylhexosaminidase in metabolism. Biochem Anal Biochem 04(04):1–2. https://doi.org/10.4172/2161-1009.1000215
Li B, Li H, Lu L, Jiang J (2017) Structures of human O-GlcNAcase and its complexes reveal a new substrate recognition mode. Nat Struct Mol Biol 24(4):362–369. https://doi.org/10.1038/nsmb.3390
Patterson MC (2014) Hexosaminidase deficiency. In: Encyclopedia of the Neurological Sciences. Elsevier Inc. 564–565. https://doi.org/10.1016/B978-0-12-385157-4.00098-1
Kim C, Nam DW, Park SY et al (2013) O-linked β-N-acetylglucosaminidase inhibitor attenuates β-amyloid plaque and rescues memory impairment. Neurobiol Aging 34(1):275–285. https://doi.org/10.1016/J.NEUROBIOLAGING.2012.03.001
Hušáková L, Riva S, Casali M et al (2001) Enzymatic glycosylation using 6-O-acylated sugar donors and acceptors: β-N-acetylhexosaminidase-catalysed synthesis of 6-O, N, N′-triacetylchitobiose and 6′-O, N, N′-triacetylchitobiose. Carbohyd Res 331(2):143–148. https://doi.org/10.1016/S0008-6215(01)00027-1
Slámová K, Bojarová P (2017) Engineered N-acetylhexosamine-active enzymes in glycoscience. Biochim et Biophysica Acta (BBA) - General Subject 8:2070–2087. https://doi.org/10.1016/j.bbagen.2017.03.019
Yan N, Chen X (2015) Sustainability: don’t waste seafood waste. Nature 524(7564):155–157. https://doi.org/10.1038/524155a
Riehle F, Hoenders D, Guo J, Eckert A, Ifuku S, Walther A (2019) Sustainable chitin nanofibrils provide outstanding flame-retardant nanopapers. Biomacromol 20(2):1098–1108. https://doi.org/10.1021/acs.biomac.8b01766
Namboodiri MMT, Pakshirajan K (2022) Valorization of waste biomass for chitin and chitosan production. In: Waste Biorefinery. Elsevier 241–266. https://doi.org/10.1016/b978-0-12-818228-4.00010-1
Kaczmarek MB, Struszczyk-Swita K, Li X, Szczęsna-Antczak M, Daroch M (2019) Enzymatic modifications of chitin, chitosan, and chitooligosaccharides. Front Bioeng Biotechnol 7(SEP):243. https://doi.org/10.3389/fbioe.2019.00243
Souza CP, Almeida BC, Colwell RR, Rivera ING (2011) The importance of chitin in the marine environment. Mar Biotechnol 13(5):823–830. https://doi.org/10.1007/s10126-011-9388-1
Stoykov YM, Pavlov AI, Krastanov AI (2015) Chitinase biotechnology: production, purification, and application. Eng Life Sci 15(1):30–38. https://doi.org/10.1002/elsc.201400173
Sato Y, Araki Y (2008) Identification of inducers for chitinase B (ChiB) production in Bacillus cereus CH and estimation of its induction mechanism. J Environ Biotechnol 8(2):119–121. Accessed June 16, 2019. https://www.jseb.jp/wordpress/wp-content/uploads/08-02-119.pdf
Sato Y, Araki Y (2007) Analysis of ChiA and ChiB production by bacillus cereus CH: induction, gene expression, and localization of two chitinases. J Environ Biotechnol 7(1):27–32. https://www.jseb.jp/wordpress/wp-content/uploads/07-01-27.pdf. Accessed 16 Jun 2019
Chen JK, Shen CR, Liu CL (2010) N-Acetylglucosamine: production and applications. Mar Drugs 8(9):2493–2516. https://doi.org/10.3390/md8092493
Hirano S, Nagao N (1988) An improved method for the preparation of colloidal chitin by using methanesulfonic acid. Agric Biol Chem 52(8):2111–2112. https://doi.org/10.1080/00021369.1988.10868977
Souza CP, Burbano-Rosero EM, Almeida BC, Martins GG, Albertini LS, Rivera ING (2009) Culture medium for isolating chitinolytic bacteria from seawater and plankton. World Journal of Microbiology and Biotechnology. 25(11):2079–2082. https://doi.org/10.1007/s11274-009-0098-z
Cardozo FA, Facchinatto WM, Colnago LA, Campana-Filho SP, Pessoa A (2019) Bioproduction of N-acetyl-glucosamine from colloidal α-chitin using an enzyme cocktail produced by Aeromonas caviae CHZ306. World J Microbiol Biotechnol 35(8):114. https://doi.org/10.1007/s11274-019-2694-x
Liu L, Liu Y, Shin H, dong, et al (2013) Microbial production of glucosamine and N-acetylglucosamine: advances and perspectives. Appl Microbiol Biotechnol 97(14):6149–6158. https://doi.org/10.1007/s00253-013-4995-6
Jung WJ, Park RD (2014) Bioproduction of chitooligosaccharides: present and perspectives. Mar Drugs 12(11):5328–5356. https://doi.org/10.3390/md12115328
Dahiya N, Tewari R, Hoondal GS (2006) Biotechnological aspects of chitinolytic enzymes: a review. Appl Microbiol Biotechnol 71(6):773–782. https://doi.org/10.1007/s00253-005-0183-7
Das S, Roy D, Sen R (2016) Utilization of chitinaceous wastes for the production of chitinase. Adv Food Nutr Res 78:27–46. https://doi.org/10.1016/bs.afnr.2016.04.001
Box GEP, Behnken DW (1960) Simplex-sum designs: a class of second order rotatable designs derivable from those of first order. Ann Math Stat 31(4):838–864. https://doi.org/10.1214/aoms/1177705661
Barbosa HR, Rodrigues MFA, Campos CC et al (1995) Counting of viable cluster-forming and non cluster-forming bacteria: a comparison between the drop and the spread methods. J Microbiol Methods 22(1):39–50. https://doi.org/10.1016/0167-7012(94)00062-C
Pirt SJ (1976) Principles of microbe and cell cultivation. Halsted Pres, Division of John Wiley and Son. https://openlibrary.org/books/OL5063023M/Principles_of_microbe_and_cell_cultivation. Accessed 23 Sept 2019
Henningsen A, Hamann JD (2007) Systemfit: a package for estimating systems of simultaneous equations in R. J Stat Software 23(4):1–40. https://doi.org/10.18637/jss.v023.i04
Keyhani NO, Roseman S (1999) Physiological aspects of chitin catabolism in marine bacteria. Biochimica et Biophysica Acta (BBA) General Subjects. 1473(1):108–122. https://doi.org/10.1016/S0304-4165(99)00172-5
Thompson FL, Neto AA, de Santos EO, Izutsu K, Iida T (2011) Effect of N-acetyl-D-glucosamine on gene expression in Vibrio parahaemolyticus. Microbes Environ 26(1):61–66. https://doi.org/10.1264/jsme2.ME10152
Meriem G, Mahmoud K (2017) Optimization of chitinase production by a new Streptomyces griseorubens C9 isolate using response surface methodology. Annal Microbiol 67(2):175–183. https://doi.org/10.1007/s13213-016-1249-8
Aounallah MA, Slimene-Debez I, ben, Djebali K, et al (2017) Enhancement of exochitinase production by Bacillus licheniformis AT6 strain and improvement of N-acetylglucosamine production. Appl Biochem Biotechnol 181(2):650–666. https://doi.org/10.1007/s12010-016-2239-9
He Y, Xu J, Wang S, Zhou G, Liu J (2014) Optimization of medium components for production of chitin deacetylase by Bacillus amyloliquefaciens Z7, using response surface methodology. Biotechnol Biotechnol Equip 28(2):242–247. https://doi.org/10.1080/13102818.2014.907659
Kim TI, Lim DH, Baek KS, Jang SS, Park BY, Mayakrishnan V (2018) Production of chitinase from Escherichia fergusonii, chitosanase from Chryseobacterium indologenes, Comamonas koreensis and its application in N-acetylglucosamine production. Int J Biol Macromolecules 112:1115–1121. https://doi.org/10.1016/J.IJBIOMAC.2018.02.056
Egli Th, Quayle JR (1986) Influence of the carbon: nitrogen ratio of the growth medium on the cellular composition and the ability of the methylotrophic yeast Hansenula polymorpha to utilize mixed carbon sources. Microbiology 132(7):1779–1788. https://doi.org/10.1099/00221287-132-7-1779
Vrede T (1998) Elemental composition (C:N:P) and growth rates of bacteria and Rhodomonas grazed by Daphnia. J Plankton Res 20(3):455–470. https://doi.org/10.1093/plankt/20.3.455
Touratierl F, Egendre’ L, Vezina A (1999) Model of bacterial growth influenced by substrate C:N ratio and concentration. Aquat Microb Ecol 19:105–118. https://pdfs.semanticscholar.org/7aed/6e0956822101a43a4999d1703c2ab667472d.pdf. Accessed 17 Jun 2019
Vrede K, Heldal M, Norland S, Bratbak G (2002) Elemental composition (C, N, P) and cell volume of exponentially growing and nutrient-limited bacterioplankton. Appl Environ Microbiol 68(6):2965–2971. https://doi.org/10.1128/aem.68.6.2965-2971.2002
Mwangi ESK, Gatebe EG, Ndung’u MW (2012) Impact of nutritional (C: N ratio and source) on growth, oxalate accumulation, and culture pH by Sclerotinia Sclerotiorum. J Bio Agri Healthcare 2(10):136–146. https://www.iiste.org/Journals/index.php/JBAH/article/view/3284/3330. Accessed 17 Jun 2019
Saima KM, Roohi AI (2013) Isolation of novel chitinolytic bacteria and production optimization of extracellular chitinase. J Gen Eng Biotechnol 11(1):39–46. https://doi.org/10.1016/J.JGEB.2013.03.001
Jami Al Ahmadi K, Tabatabaei Yazdi M, Fathi Najafi M et al (2008) Optimization of medium and cultivation conditions for chitinase production by the newly isolated: Aeromonas sp. Biotechnology 7(2):266–272. https://doi.org/10.3923/biotech.2008.266.272
Ghasemi Y, Dehdari Z, Mohkam M, Kargar M (2013) Isolation and optimization of cultivation conditions for production of chitinase by Aeromonas sp. ZD_05 from the Persian Gulf. J Pure App Microbiol 7:913–918. https://microbiologyjournal.org/archive_mg/jmabsread.php?snoid=1226&month=&year=. Accessed 17 Jun 2019
Palumbro SA, Morgan DR, Buchanan RL (1985) Influence of temperature, NaCI, and pH on the growth of Aeromonas Hydrophila. J Food Sci 50(5):1417–1421. https://doi.org/10.1111/j.1365-2621.1985.tb10490.x
Häunninen ML (1994) Phenotypic characteristics of the three hybridization groups of Aeromonas hydrophila complex isolated from different sources. J Appl Bacteriol 76(5):455–462. https://doi.org/10.1111/j.1365-2672.1994.tb01102.x
Shanmugaiah V, Mathivanan N, Balasubramanian N, Manoharan PT (2008) Optimization of cultural conditions for production of chitinase by Bacillus laterosporous MML2270 isolated from rice rhizosphere soil. African J Biotechnol 7(15):2562–2568. http://www.academicjournals.org/AJB. Accessed 17 Jun 2019
Gomaa EZ (2012) Chitinase production by Bacillus thuringiensis and Bacillus licheniformis: their potential in antifungal biocontrol. J Microbiol 50(1):103–111. https://doi.org/10.1007/s12275-012-1343-y
Itoh T, Hibi T, Fujii Y et al (2013) Cooperative degradation of chitin by extracellular and cell surface-expressed chitinases from Paenibacillus sp. Strain FPU-7. Appl Environ Microbiol 79(23):7482–7490. https://doi.org/10.1128/AEM.02483-13
Garcia-Ochoa F, Gomez E (2009) Bioreactor scale-up and oxygen transfer rate in microbial processes: an overview. Biotechnol Adv 27(2):153–176. https://doi.org/10.1016/J.BIOTECHADV.2008.10.006
Rosa JC, Neto AB, Hokka CO, Badino AC (2005) Influence of dissolved oxygen and shear conditions on clavulanic acid production by Streptomyces clavuligerus. Bioprocess Biosyst Eng 27(2):99–104. https://doi.org/10.1007/s00449-004-0386-9
Baez A, Shiloach J (2014) Effect of elevated oxygen concentration on bacteria, yeasts, and cells propagated for production of biological compounds. Microb Cell Fact 13:181. https://doi.org/10.1186/S12934-014-0181-5
Kao PM, Chen CI, Huang SC, Chang YC, Tsai PJ, Liu YC (2007) Effects of shear stress and mass transfer on chitinase production by Paenibacillus sp CHE-N1. Bioche Eng J 34(2):172–178. https://doi.org/10.1016/J.BEJ.2006.11.028
Chaiharn M, Lumyong S, Hasan N, Plikomol A (2013) Solid-state cultivation of Bacillus thuringiensis R 176 with shrimp shells and rice straw as a substrate for chitinase production. Annal Microbiol 63(2):443–450. https://doi.org/10.1007/s13213-012-0488-6
Cha JM, Cheong KH, Cha WS, Choi D, Roh SH, Kim S, ll, (2004) Optimal conditions for chitinase production by Serratia marcescens. Biotechnol Bioproc Eng 9(4):297–302. https://doi.org/10.1007/BF02942347
Gupta R, Saxena RK, Chaturvedi P, Virdi JS (1995) Chitinase production by Streptomyces viridificans: its potential in fungal cell wall lysis. J Appl Bacteriol 78(4):378–383. http://www.ncbi.nlm.nih.gov/pubmed/7744723. Accessed 17 Jun 2019
Box GEP, Hunter WG, Hunter JS (1978) Statistics for Experimenters: An Introduction to Design, Data Analysis, and Model Building. John Wiley & Sons, New Jersey
Tews I, Perrakis A, Oppenheim A, Dauter Z, Wilson KS, Vorgias CE (1996) Bacterial chitobiase structure provides insight into catalytic mechanism and the basis of Tay-Sachs disease. Nat Struct Biol 3(7):638–648. https://doi.org/10.1038/nsb0796-638. (PMID: 8673609)
Funding
This work was supported by the Sao Paulo Research Foundation (FAPESP), Brazil, [grant numbers 2012/16824–0 and 2013/18773–6].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Flávio Augusto Cardozo and Valker Araujo Feitosa shared first co-author.
Responsible Editor: Solange I. Mussatto
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cardozo, F.A., Feitosa, V., Mendonça, C.M.N. et al. Enhanced production of N-acetyl-glucosaminidase by marine Aeromonas caviae CHZ306 in bioreactor. Braz J Microbiol 54, 1533–1545 (2023). https://doi.org/10.1007/s42770-023-01088-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-01088-x