Abstract
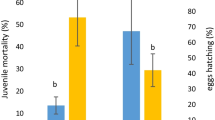
In the present study, the nematicidal and acaricidal activity of three biosurfactants (BS) produced by strains of the Bacillus genus was evaluated. The BS produced by the Bacillus ROSS2 strain presented a mortality of 39.29% in juveniles (J2) of Nacobbus aberrans at a concentration of 30 mg/mL, this same strain is the one that presented the highest mortality in Tyrophagus putrescentiae, which was 57.97% at a concentration of 39 mg/mL. The BS were qualitatively identified by thin layer chromatography and are lipid in nature based on the retention factor (Rf). While the GC-MS analysis identified two main compounds that are 4,7-Methano-1H-indene-2,6-dicarboxylic acid, 3a,4,7,7a-tetrahydro-1, and Methyl 4-(pyrrol-1-yl)-1,2,5-oxadiazole-3-carboxylate1, which is the polar part indicated by the presence of dicarboxylic acid and carboxylate groups; while the non-polar portion can be interpreted as a hydrocarbon chain of variable length. Based on the present results, BS can be an alternative for the biocontrol of the root-knot nematode N. aberrans and the mite T. putrescentiae.



Similar content being viewed by others
References
Manzanilla LRH, Costilla MA, Doucet M, Inserra RN, Lehman PS, Cid del PV, Souza RM, Evans K (2002) The genus Nacobbus Thorne & Allen, 1944 (Nematoda: Pratylenchidae): systematics, distribution, biology and management. Nematropica 32:149–227
Méndez SEW, Sánchez CR, Folch MJL, Aguilar ML, Hernández VVM, Gómez RO, Villar LE, Wong VA (2020) Serratia sp., an endophyte of Mimosa pudica nodules with nematicidal, antifungal activity and growth promoting characteristics. Arch Microbiol https://doi.org/10.1007/s00203-020-02051-2
Hughes AM (1976) The mites of stored food and houses her majesty’s stationery office, London, UK
Colloff MJ (2009) Dust mite allergens. In Dust mites, Springer, Dordrecht, p 2009
Duek L, Kaufman G, Palevsky E, Berdicevsky I (2001) Mites in fungal cultures. Mycoses 44(9-10):390–394. https://doi.org/10.1046/j.1439-0507.2001.00684.x
Erban T, Klimov PB, Smrz J, Phillips TW, Nesvorna M, Kopecky J, Hubert J (2016) Populations of stored product mite Tyrophagus putrescentiae differ in their bacterial communities. Front Microbiol 12(7):1046. https://doi.org/10.3389/fmicb.2016.01046
Erban T, Ledvinka O, Nesvorna M, Hubert J (2017) Experimental manipulation shows a greater influence of population than dietary perturbation on the microbiome of Tyrophagus putrescentiae. Appl Environ Microbiol 83(9):e00128–e00117. https://doi.org/10.1128/AEM.00128-17
de Andrade TFN, de Souza AC, Simões LA, Ferreira Dos RGM, Souza KT, Schwan RF, Dias DR (2020) Eco-friendly biosurfactant from Wickerhamomyces anomalus CCMA 0358 as larvicidal and antimicrobial. Microbiol Res 241:126571. https://doi.org/10.1016/j.micres.2020.126571
Al-Assiuty BA, Nenaah GE, Ageba ME (2019) Chemical profile, characterization and acaricidal activity of essential oils of three plant species and their nanoemulsions against Tyrophagus putrescentiae, a stored food mite. Exp Appl Acarol 79(3-4):359–376. https://doi.org/10.1007/s10493-019-00432-x
Kaya D, Inceboz T, Kolatan E, Güneli E, Yilmaz O (2010) Comparison of efficacy of ivermectin and doramectin against mange mite (Sarcoptes scabiei) in naturally infested rabbits in Turkey. Vet Ital 46(1):51–56
Tak JH, Kim HK, Lee SH, Ahn YJ (2006) Acaricidal activities of paeonol and benzoic acid from Paeonia suffruticosa root bark and monoterpenoids against Tyrophagus putrescentiae (Acari: Acaridae). Pest Manag Sci 62(6):551–557. https://doi.org/10.1002/ps.1212
Marcelino PRF, Gonçalves F, Jimenez IM, Carneiro BC, Santos BB, da Silva SS (2020) Sustainable production of biosurfactants and their applications. In: Ingle AP, Chandel AK, da Silva SS (eds) Lignocellulosic Biorefining Technologies. John Wiley & Sons Ltd, Pondicherry, India, pp 159–184
Varjani SJ, Upasani VN (2017) Critical review on biosurfactant analysis, purification and characterization using rhamnolipid as a model biosurfactant. Bioresour Technol 232:389–397. https://doi.org/10.1016/j.biortech.2017.02.047
Kubicki S, Bollinger A, Katzke N, Jaeger KE, Loeschcke A, Thies S (2019) Marine biosurfactants: biosynthesis, structural diversity and biotechnological applications. Mar Drugs 17:1–30. https://doi.org/10.3390/md17070408
Varjani SJ, Upasani VN (2019) Evaluation of rhamnolipid production by a halotolerant novel strain of Pseudomonas aeruginosa. Bioresour Technol 288:121577. https://doi.org/10.1016/j.biortech.2019.121577
Markandev AR, Patel D, Varjani S (2021) A review on biosurfactants: properties, applications and current developments. Bioresour Technol 330:124963. https://doi.org/10.1016/j.biortech.2021.124963
D’aes J, De Maeyer K, Pauwelyn E, Höfte M (2010) Biosurfactants in plant–Pseudomonas interactions and their importance to biocontrol. Environ Microbiol Rep 2(3):359–372
Adnan M, Siddiqui AJ, Hamadou WS, Ashraf SA, Hassan MI, Snoussi M, Patel M (2021) Functional and structural characterization of Pediococcus pentosaceus derived biosurfactant and its biomedical potential against bacterial adhesion, quorum sensing, and biofilm formation. Antibiot 10(11):1371
Patel M, Siddiqui AJ, Hamadou WS, Surti M, Awadelkareem AM, Ashraf SA, Adnan M (2021) Inhibition of bacterial adhesion and antibiofilm activities of a glycolipid biosurfactant from Lactobacillus rhamnosus with its physicochemical and functional properties. Antibiot 10(12):1546
Fazaeli N, Bahador N, Hesami S (2021) A study on larvicidal activity and phylogenetic analysis of Staphylococcus epidermidis as a biosurfactant-producing bacterium. Pol J Environ Stud 30(5):4511–4519. https://doi.org/10.15244/pjoes/132807
Franco MPR, da Silva VL, Rodrigues PR, Von Zuben CJ, Contiero J, dos Santos JC (2017) Biosurfactants produced by Scheffersomyces stipitis cultured in sugarcane bagasse hydrolysate as new green larvicides for the control of Aedes aegypti, a vector of neglected tropical diseases. PLoS One 12(11):e0187125. https://doi.org/10.1371/journal.pone.0187125
Hussain T, Haris M, Shakeel A (2020) Bio-nematicidal activities by culture filtrate of Bacillus subtilis HussainT-AMU: new promising biosurfactant bioagent for the management of Root Galling caused by Meloidogyne incognita. Vegetos 33:229–238. https://doi.org/10.1007/s42535-020-00099-5
Kim SK, Kim YC, Lee S, Kim JC, Yun MY, Kim IS (2011) Insecticidal activity of rhamnolipid isolated from Pseudomonas sp. EP-3 against green peach aphid (Myzus persicae). J Agric Food Chem 59(3):934–938. https://doi.org/10.1021/jf104027x
Vrain TC (1997) A technique for the collection of larvae of Meloidogyne spp. and a comparison of eggs and larvae as inoculate. J Nematol 9:249–251. https://doi.org/10.1016/j.jip.2006.03.006
Villar LE, Reyes TB, Rojas MR, Gómez RO, Hernández AA, Zavaleta ME (2009) Respuesta hipersensitiva en el follaje de chile CM.334 resistente a Phytophthora capsici infectado con Nacobbus aberrans. Nematropica 39:143–155
Aguilar ML, Quintero MMT, De Gives PM, López AME, Liébano HE, Torres HG, Del Prado IC (2014) Evaluation of predation of the mite Lasioseius penicilliger (Aracnida: Mesostigmata) on Haemonchus contortus and bacteria feeding nematodes. J Helminthol 88(1):20–23
De Lara R, Castro T, Castro J, Castro G (2007) Nematode culture of Panagrellus redivivus (Goodey, 1945) with Spirulina sp., enriched oatmeal. Rev Biol Mar Oceanogr 42:29–36
Weisburg GW, Barns MS, Pelletier AD, Lane JD (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–603
Gouy M, Guindon S, Gascuel O (2010) SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27(2):221–224. https://doi.org/10.1093/molbev/msp259
Santos DK, Rufino RD, Luna JM, Santos VA, Salgueiro AA, Sarubbo LA (2013) Synthesis and evaluation of biosurfactant produced by Candida lipolytica using animal fat and corn steep liquor. J Pet Sci Eng 105:43–50
Silva EJ, NMPR ES, Rufino RD, Luna JM, Silva RO, Sarubbo LA (2014) Characterization of a biosurfactant produced by Pseudomonas cepacia CCT6659 in the presence of industrial wastes and its application in the biodegradation of hydrophobic compounds in soil. Colloids and Surf. B: Biointerfaces 117:36–41
Hahn MH, De Mio LLM, Kuhn OJ, Duarte HDSS (2019) Nematophagous mushrooms can be an alternative to control Meloidogyne javanica. Biol Control 138:104024
Pineda AJA, Sánchez VJE, Gonzalez CM, Zamilpa A, López AME, Cuevas PEJ, Aguilar ML (2017) The edible mushroom Pleurotus djamor produces metabolites with lethal activity against the parasitic nematode Haemonchus contortus. J Med Food 20(12):1184–1192
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 27 May 2022
Ibrahim ML, Ijah UJJ, Manga SB, Bilbis LS, Umar S (2013) Production and partial characterization of biosurfactant produced by crude oil degrading bacteria. Int Biodeter Biodegr 81:28–34
Ismail W, Al-Rowaihi IS, Al-Humam AA, Hamza RY, El Nayal AM (2013) Bououdina, M. Characterization of a lipopeptide biosurfactant produced by a crude-oil-emulsifying Bacillus sp. I-15. Int Biodeter Biodegr 84:168–178
Kuyukina MS, Ivshina IB, Philp JC, Christofi N, Dunbar SA, Ritchkova MI (2001) Recovery of Rhodococcus biosurfactants using methyl tertiary-butyl ether extraction. J Microbiol Methods 46(2):149–156
Janek T, Łukaszewicz M, Krasowska A (2013) Identification and characterization of biosurfactants produced by the arctic bacterium Pseudomonas putida BD2. Colloids Surf B Biointerfaces 110:379–386
Tuleva B, Christova N, Jordanov B, Nikolova-Damyanova B, Petrov P (2005) Naphthalene degradation and biosurfactant activity by Bacillus cereus 28BN. Z Naturforsch C J Biosci 60(7-8):577–582. https://doi.org/10.1515/znc-2005-7-811
Durval IJB, Mendonça AHR, Rocha IV, Luna JM, Rufino RD Converti A, Sarubbo LA (2020) Production, characterization, evaluation and toxicity assessment of a Bacillus cereus UCP 1615 biosurfactant for marine oil spills bioremediation. Mar Pollut Bull 157:111357. https://doi.org/10.1016/j.marpolbul.2020.111357.
Bhawsar SD, Path SD, Chopade BA (2011) Antimicrobial activity of purified emulsifier of acinetobacter purified genospecies isolated from rhizosphere of wheat. Agric Sci Dig 31:239–246
Naughton PJ, Marchant R, Naughton V, Banat IM (2019) Microbial biosurfactants: current trends and applications in agricultural and biomedical industries. J Appl Microbiol 127:12–28. https://doi.org/10.1111/jam.14243
Shaikh S, Yadav N, Markande AR (2020) Interactive potential of Pseudomonas species with plants. J Appl Biol Biotechnol 8:101–111. https://doi.org/10.7324/jabb.2020.80616
Kumar A, Singh SK, Kant C, Verma H, Kumar D, Singh PP, Modi A, Droby S, Kesawat MS, Alavilli H (2021) Microbial biosurfactant: a new frontier for sustainable agriculture and pharmaceutical industries. Antioxidants 10:1472. https://doi.org/10.3390/antiox10091472
Geetha I, Manonmani AM (2010) Surfactin: a novel mosquitocidal biosurfactant produced by Bacillus subtilis ssp. subtilis (VCRC B471) and influence of abiotic factors on its pupicidal efficacy. Lett Appl Microbiol 4:406–412. https://doi.org/10.1111/j.1472-765X.2010.02912.x
Curtis D Thompson B (2016) Compositions comprising recombinant Bacillus cells and another biological control agent. International application published under the patent cooperation treaty (PCT) WO2016044529-A1.
Płaza G, Chojniak J, Rudnicka K, Paraszkiewicz K, Bernat P (2015) Detection of biosurfactants in Bacillus species: genes and products identification. J Appl Microbiol 119(4):1023–1034. https://doi.org/10.1111/jam.12893
Nawazish A, Zhengjun P, Fenghuan W, Baocai X, Hesham R, El-S (2022) Lipopeptide biosurfactants from Bacillus spp.: types, production, biological activities, and applications in food. J Food Qual. https://doi.org/10.1155/2022/3930112
Acknowledgements
The authors thank M.C. Susan Yaracet Páez-León and IBT. Angelita Morales-Morales for assisting with the research. This study was partially financed by the Fiscal Resources Project of INIFAP with the number: 139335341.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Enderson Ferreira
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gómez-Gutiérrez, J.A., Wong-Villarreal, A., Aguilar-Marcelino, L. et al. In vitro nematicidal and acaricidal effect of biosurfactants produced by Bacillus against the root-knot nematode Nacobbus aberrans and the dust mite Tyrophagus putrescentiae. Braz J Microbiol 54, 1127–1136 (2023). https://doi.org/10.1007/s42770-023-00981-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-00981-9