Abstract
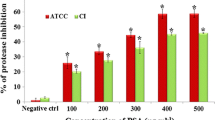
Quorum sensing (QS) is an inter- and intracellular communication mechanism that regulates gene expression in response to population size. Autoinducer-2 (AI-2) signaling is a QS signaling molecule common to both Gram-negative and Gram-positive bacteria. Enterococcus faecalis is one of the leading causes of nosocomial infections worldwide. There has been an increasing interest in controlling infectious diseases through targeting the QS mechanism using natural compounds. This study aimed to investigate the effect of nisin and p-coumaric acid (pCA), on biofilm formation and AI-2 signaling in E. faecalis. Their effect on the expression of the QS-regulated virulence encoding gene sprE was also investigated. Nisin exhibited a MIC ranging from 0.25 to 0.5 mg/mL, while the MIC of pCA was 1 mg/mL. The luminescence-based response of the reporter strain Vibrio harveyi BB170 was used to determine AI-2 activity in E. faecalis strains. Nisin was not effective in inhibiting AI-2 activity, while pCA reduced AI-2 activity by ≥ 60%. Moreover, pCA and nisin combination showed higher inhibitory effect on biofilm formation of E. faecalis, compared to the treatment of pCA or nisin alone. qRT-PCR analysis showed that nisin alone and the combination of nisin and pCA, at their MIC values, led to a 32.78- and 40.22-fold decrease in sprE gene expression, respectively, while pCA alone did not have a significant effect. Considering the demand to explore new therapeutic avenues for infectious bacteria, this study was the first to report that pCA can act like a quorum sensing inhibitor (QSI) against AI-2 signaling in E. faecalis.




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Miller MB, Bassler BL (2001) Quorum sensing in bacteria. Annu Rev Microbiol 55:165–199
Ng WL, Bassler BL (2009) Bacterial quorum-sensing network architectures. Annu Rev Genet 43:197–222
Nazzaro F, Fratianni F, Coppola R (2013) Quorum sensing and phytochemicals. Int J Mol Sci 14(6):12607–12619
Waters CM, Bassler BL (2005) Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol 21:319–346
Bassler BL (1999) How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr Opin Microbiol 2(6):582–587
Kendall MM, Sperandio V (2014) Cell-to-cell signaling in Escherichia coli and Salmonella. EcoSal Plus 6(1). https://doi.org/10.1128/ecosalplus.ESP-0002-2013
Pereira CS, Thompson JA, Xavier KB (2013) AI-2-mediated signalling in bacteria. FEMS Microbiol Rev 37(2):156–181
Xavier KB, Bassler BL (2005) Interference with AI-2-mediated bacterial cell-cell communication. Nature 437(7059):750–753
Schauder S et al (2001) The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol Microbiol 41(2):463–476
Richards MJ et al (2000) Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol 21(8):510–515
Haghi F, Lohrasbi V, Zeighami H (2019) High incidence of virulence determinants, aminoglycoside and vancomycin resistance in enterococci isolated from hospitalized patients in Northwest Iran. BMC Infect Dis 19(1):744
Dunny GM, LE Hancock, Shankar N (2014) Enterococcal biofilm structure and role in colonization and disease. In: Gilmore MS et al. (eds) Enterococci: from commensals to leading causes of drug resistant infection. Massachusetts Eye and Ear Infirmary, Boston
Mohamed JA, Huang DB (2007) Biofilm formation by enterococci. J Med Microbiol 56(Pt 12):1581–1588
Costerton JW, Stewart PS, Greenberg E (1999) Bacterial biofilms: a common cause of persistent infections. Science 284(5418):1318–1322
Jiang Q et al (2019) Quorum sensing: a prospective therapeutic target for bacterial diseases. Biomed Res Int 2019:2015978
Santhakumari S, Ravi AV (2019) Targeting quorum sensing mechanism: an alternative anti-virulent strategy for the treatment of bacterial infections. S Afr J Bot 120:81–86
Hentzer M, Givskov M (2003) Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J Clin Invest 112(9):1300–1307
Kalia VC, Raju SC, Purohit HJ (2011) Genomic analysis reveals versatile organisms for quorum quenching enzymes: acyl-homoserine lactone-acylase and -lactonase. Open Microbiol J 5:1–13
Shin JM et al (2016) Biomedical applications of nisin. J Appl Microbiol 120(6):1449–1465
Gharsallaoui A et al (2016) Nisin as a food preservative: part 1: physicochemical properties, antimicrobial activity, and main uses. Crit Rev Food Sci Nutr 56(8):1262–1274
Prince A et al (2016) Lipid-II independent antimicrobial mechanism of nisin depends on its crowding and degree of oligomerization. Sci Rep 6:37908
Oshima S et al (2014) Nisin A extends the shelf life of high-fat chilled dairy dessert, a milk-based pudding. J Appl Microbiol 116(5):1218–1228
Joo NE et al (2012) Nisin, an apoptogenic bacteriocin and food preservative, attenuates HNSCC tumorigenesis via CHAC1. Cancer Med 1(3):295–305
Tong Z et al (2014) An in vitro study on the effects of nisin on the antibacterial activities of 18 antibiotics against Enterococcus faecalis. PLoS ONE 9(2):e89209
Mathur H et al (2018) Fighting biofilms with lantibiotics and other groups of bacteriocins. NPJ Biofilms Microbiomes 4:9
Ferreira PS et al (2019) A review of analytical methods for p-coumaric acid in plant-based products, beverages, and biological matrices. Crit Rev Anal Chem 49(1):21–31
Abdel-Wahab MH et al (2003) Influence of p-coumaric acid on doxorubicin-induced oxidative stress in rat’s heart. Pharmacol Res 48(5):461–465
Pei K et al (2016) p-Coumaric acid and its conjugates: dietary sources, pharmacokinetic properties and biological activities. J Sci Food Agric 96(9):2952–2962
Lou Z et al (2012) p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 25(2):550–554
Myszka K et al (2016) Role of gallic and p-coumaric acids in the AHL-dependent expression of flgA gene and in the process of biofilm formation in food-associated Pseudomonas fluorescens KM120. J Sci Food Agric 96(12):4037–4047
Kot B et al (2015) Antibiofilm activity of trans-cinnamaldehyde, p-coumaric, and ferulic acids on uropathogenic Escherichia coli. Turk J Med Sci 45(4):919–924
Kitazaki K et al (2017) In vitro synergistic activities of cefazolin and nisin A against mastitis pathogens. J Vet Med Sci 79(9):1472–1479
Jesudhasan PR et al (2010) Transcriptome analysis of genes controlled by luxS/autoinducer-2 in Salmonella enterica serovar Typhimurium. Foodborne Pathog Dis 7(4):399–410
Lu L, Hume ME, Pillai SD (2004) Autoinducer-2-like activity associated with foods and its interaction with food additives. J Food Prot 67(7):1457–1462
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3(6):1101–1108
Anderson AC et al (2015) Enterococcus faecalis from food, clinical specimens, and oral sites: prevalence of virulence factors in association with biofilm formation. Front Microbiol 6:1534
Zhao X, Z Yu, Ding T (2020) Quorum-sensing regulation of antimicrobial resistance in bacteria. Microorganisms 8(3):425
Taofiq O et al (2019) Phenolic acids, cinnamic acid, and ergosterol as cosmeceutical ingredients: stabilization by microencapsulation to ensure sustained bioactivity. Microchem J 147:469–477
Grenier D et al (2020) Biocompatible combinations of nisin and licorice polyphenols exert synergistic bactericidal effects against Enterococcus faecalis and inhibit NF-kappaB activation in monocytes. AMB Express 10(1):120
Bodini SF et al (2009) Quorum sensing inhibition activity of garlic extract and p-coumaric acid. Lett Appl Microbiol 49(5):551–555
Gui M et al (2021) Effect of AHL-lactonase and nisin on microbiological, chemical and sensory quality of vacuum packaged sturgeon storage at 4ºC. Int J Food Prop 24(1):222–232
Bag A, Chattopadhyay RR (2017) Synergistic antibacterial and antibiofilm efficacy of nisin in combination with p-coumaric acid against food-borne bacteria Bacillus cereus and Salmonella typhimurium. Lett Appl Microbiol 65(5):366–372
Engelbert M et al (2004) Contribution of gelatinase, serine protease, and fsr to the pathogenesis of Enterococcus faecalis endophthalmitis. Infect Immun 72(6):3628–3633
Qin X et al (2000) Effects of Enterococcus faecalis fsr genes on production of gelatinase and a serine protease and virulence. Infect Immun 68(5):2579–2586
Qin X et al (2001) Characterization of fsr, a regulator controlling expression of gelatinase and serine protease in Enterococcus faecalis OG1RF. J Bacteriol 183(11):3372–3382
Ahmed S et al (2019) Natural quorum sensing inhibitors effectively downregulate gene expression of Pseudomonas aeruginosa virulence factors. Appl Microbiol Biotechnol 103(8):3521–3535
Funding
This work was financially supported by the Scientific and Technological Research Council of Turkey (grant 118Z697). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Ilana Camargo
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yazıcı, B.C., Bakhedda, N. & Akçelik, N. Effect of nisin and p-coumaric acid on autoinducer-2 activity, biofilm formation, and sprE expression of Enterococcus faecalis. Braz J Microbiol 54, 601–608 (2023). https://doi.org/10.1007/s42770-023-00946-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-00946-y