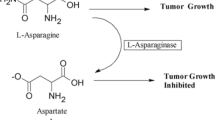
Abstract
l-Asparaginase (l-ASNase) is a potent chemotherapeutic drug employed to treat leukemia and lymphoma. Currently, l-ASNases for therapeutic use are obtained from Escherichia coli and Dickeya chrysanthemi (Erwinia chrysanthemi). Despite their therapeutic potential, enzymes from bacteria are subject to inducing immune responses, resulting in a higher number of side effects. Eukaryote producers, such as fungi, may provide therapeutic alternatives through enzymes that induce relatively less toxicity and immune responses. Additional expected benefits from yeast-derived enzymes include higher activity and stability in physiological conditions. This work describes the new potential therapeutic candidate l-ASNase from the yeast Meyerozyma guilliermondii. A statistical approach (full factorial central composite design) was used to optimize l-ASNase production, considering l-asparagine and glucose concentration, pH of the medium, and cultivation time as independent factors. In addition, the crude enzymes were biochemically characterized, in terms of temperature and optimal pH, thermostability, pH stability, and associated glutaminase or urease activities. Our results showed that enzyme production increased after supplementing a pH 4.0 medium with 1.0% l-asparagine and 0.5% glucose during 75 h of cultivation. Under these optimized conditions, l-ASNase production reached 26.01 U mL−1, which is suitable for scale-up studies. The produced l-ASNase exhibits maximal activity at 37 °C and pH 7.0 and is highly stable under physiological conditions. In addition, M. guilliermondii l-ASNase has no associated glutaminase or urease activities, demonstrating its potential as a promising antineoplastic agent.


Similar content being viewed by others
References
de la Fuente M, Lombardero L, Gómez-González A, Solari C, Angulo-Barturen I, Acera A, Vecino E, Astigarra E (2021) Enzyme therapy: current challenges and future perspectives. Int J Mol Sci 22:9181. https://doi.org/10.3390/ijms22179181
Vellard M (2003) The enzyme as drug: application of enzymes as pharmaceuticals. Curr Opin Biotechnol 14:444–450. https://doi.org/10.1016/s0958-1669(3)00092-2
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Sharma D, Singh K, Singh K, Mishra A (2019) Insights into the microbial l-asparaginases: from production to practical applications. Curr Protein Pept Sci 20:452–464. https://doi.org/10.2174/1389203720666181114111035
Douer D, Gökbuget N, Stock W, Boissel N (2021) Optimizing use of L-asparaginase–based treatment of adults with acute lymphoblastic leukemia. Blood Rev 2021:100908. https://doi.org/10.1016/j.blre.2021.100908
Vimal A, Kumar A (2017) Biotechnological production and practical application of L-asparaginase enzyme. Biotechnol Genet Eng Rev 33:40–61. https://doi.org/10.1080/02648725.2017.1357294
da Cunha MC, Aguilar JGS, de Melo RR, Nagamatsu ST, Ali F, de Castro RJS, Sato HH (2019) Fungal L-asparaginase: strategies for production and food applications. Food Res Int 126:108658. https://doi.org/10.1016/j.foodres.2019.108658
Brumano LP, da Silva FVS, Costa-Silva TA, Apolinário AC, Santos JHPM, Kleingesinds EK, Monteiro G, Rangel-Yagui CO, Benyahia B, Junior AP (2019) Development of L-asparaginase biobetters: current research status and review of the desirable quality profiles. Front Bioeng Biotechnol 10(6):212. https://doi.org/10.3389/fbioe.2018.00212
Izadpanah F, Homaei A, Fernandes P, Javadpour S (2018) Marine microbial L-asparaginase: biochemistry, molecular approaches and applications in tumor therapy and in food industry. Microbiol Res 208:99–112. https://doi.org/10.1016/j.micres.2018.01.011
Saeed H, Ali H, Soudan H, Embaby A, El-Sharkawy A, Farag A, Hussein A, Ataya F (2018) Molecular cloning, structural modeling and production of recombinant Aspergillus terreus asparaginase in Escherichia coli. Int J Biol Macromol 106:1041–1051. https://doi.org/10.1016/j.ijbiomac.2017.08.110
Andrade AF, Borges KS, Silveira VS (2014) Update on the use of L-asparaginase in infants and adolescent patients with acute lymphoblastic leukemia. Clin Med Insights Oncol 8:95–100. https://doi.org/10.4137/CMO.S1024
Parmentier JH, Maggi M, Tarasco E, Scotti C, Abramis VI, Mittelman SD (2015) Glutaminase activity determines cytotoxicity of l-asparaginases on most leukemia cell lines. Leuk Res 39:757–762. https://doi.org/10.1016/j.leukres.2015.04.008
Freitas M, Souza P, Cardoso S, Cruvinel K, Abrunhosa LS, Ferreira-Filho EX, Inácio J, Pinho DB, Pessoa A, Magalhães PO (2021) Filamentous fungi producing L-asparaginase with low glutaminase activity isolated from Brazilian savanna soil. Pharmaceutics 13:1268. https://doi.org/10.3390/pharmaceutics13081268
Doriya K, Kumar DS (2016) Isolation and screening of l-asparaginase free of glutaminase and urease from fungal sp. 3 Biotech 6:239. https://doi.org/10.1007/s13205-016-0544-1
Ahmed MMA, Dahab NA, Taha T, Hassan F (2015) Purification and characterization of l-asparaginase from marine endophytic Aspergillus sp. Alaa-2000 under submerged and solid state fermentation. J Microb Biochem Technol 7:165–172. https://doi.org/10.4172/1948-5948.1000199
Demain AL, Vaishnav P (2011) Production of recombinant proteins by microbes and higher organisms. Compr Biotechnol Second Ed 3:333–345. https://doi.org/10.1016/j.biotechadv.2009.01.008
Shrivastava A, Arifkhan A, Khurshid M, Abulkalam M, Jain SK, Singhal PK (2016) Recent developments in l-asparaginase discovery and its potential as anticancer agent. Crit Rev Oncol Hematol 100:1–10. https://doi.org/10.1016/j.critrevonc.2015.01.002
Cachumba JJM, Antunes FAF, Peres GFD, Brumano LP, Santos JC, Silva SS (2016) Current applications and different approaches for microbial l-asparaginase production. Braz J Microbiol 47:77–85. https://doi.org/10.1016/j.bjm.2016.10.004
Costa IM, Schultz L, Pedra BAB, Leite MSM, Farsky SHP, Oliveira MA, Pessoa A, Monteiro G (2016) Recombinant L-asparaginase 1 from Saccharomyces cerevisiae: an allosteric enzyme with antineoplastic activity. Sci Rep 6:36239. https://doi.org/10.1038/srep36239
Ashok A, Kumar DS (2021) Laboratory scale bioreactor studies on the production of L-asparaginase using Rhizopusmicrosporus IBBL-2 and Trichosporonasahii IBBLA1. Biocatal Agric Biotecnol 34:102041. https://doi.org/10.1016/j.bcab.2021.102041
Arumugam N, Tahgavelu P (2022) Purification and anticancer activity of glutaminase and urease free intracellular L-asparaginase from Chaetomium sp. Protein Expr Purif 190:106006. https://doi.org/10.1016/j.pep.2021.106006
Ashok A, Doriya K, Rao JV, Qureshi A, Tiwari AK, Kumar DS (2019) Microbes producing L-asparaginase free of glutaminase and urease activity isolated from extreme locations of Antarctic soil and moss. Sci Rep 9:1423. https://doi.org/10.1038/s41598-018-38094-1
Knob A, Izidoro SC, Lacerda LT, Rodrigues A, Lima VA (2020) A novel lipolytic yeast Meyerozymaguilliermondii: efficient and low-cost production of acid and promising feed lipase using cheese whey. Biocatal Agric Biotechnol 24:101565. https://doi.org/10.1016/j.bcab.2020.101565
Vogel HJ (1956) A convenient growth medium for (medium N). Microb Genet Bull 13:42–43
Wade HE, Phillips BP (1971) Automated determination of bacterial asparaginase and glutaminase. Anal Biochem 44(189):199. https://doi.org/10.1016/0003-2697(71)90360-5
Freire RKB, Mendonça CMN, Ferraro RB, Moguel IS, Tonso A, Lourenço FR, Santos JHPM, Sette LD, Junior AP (2021) Glutaminase-free L-asparaginase production by Leucosporidiummuscorum isolated from Antarctic marine-sediment. Prep Biochem Biotechnol 51:277–288. https://doi.org/10.1080/10826068.2020.1815053
Hymavathi M, Sathish T, SubbaRao CH, Prakasham RS (2009) Enhancement of L-asparaginase production by isolated Bacillus circulans (MTCC 8574) using response surface methodology. Appl Biochem Biotechnol 159:191–198. https://doi.org/10.1007/s12010-008-8438-2
Saleena SK, Johnson JI, Joseph JK, Padinchati KK, Abdulla MHA (2023) Production and optimization of l-asparaginase by Streptomyces koyangensis SK4 isolated from Arctic sediment. J Basic Microbiol 63:417–426. https://doi.org/10.1002/jobm.202200116
Darvishi F, Faraji N, Shamsi F (2019) Production and structural modeling of a novel asparaginase in Yarrowialipolytica. Int J Biol Macromol 125:955–961. https://doi.org/10.1016/j.ijbiomac.2018.12.162
Baskar G, Renganathan S (2011) Statistical and evolutionary optimization of operating conditions for enhanced production of fungal L-asparaginase. Chem Pap 65:798–804. https://doi.org/10.2478/s11696-011-0072-8
Pallem C, Nagarjun V, Srikanth M. Production of a tumour inhibitory enzyme, L-asparaginase through solid state fermentation using Fusarium oxysporum. Int J Pharm Sci Rev Res 7:189–192.
Thirunavukkarasu N, Suryanarayanan TS, Murali TS, Ravishankar JP, Gummadi SN (2011) L-Asparaginase from marine derived fungal endophytes of seaweeds. Mycosphere 2:147–155
Arumugam N, Shanmugam MK, Thangavelu P (2021) Purification and anticancer activity of glutaminase and urease-free-L-asparaginase from novel endophyte Chaetomium sp. Biotechnol Appl Biochem 00:1–15. https://doi.org/10.1002/bab.2276
Momeni V, Alemzadeh I, Vosoughi M (2015) Enhancement of L-asparaginase production by Candida utilis in a 13 L fermenter and its purification. Int J Eng Trans B Appl 28:1134–1139
Sarquis MI, Oliveira EM, Santos AS, Costa GL (2004) Production of L-asparaginase by filamentous fungi. Mem Inst Oswaldo Cruz 99:489–492. https://doi.org/10.1590/s0074-02762004000500005
Indira K, Jayaprabha N, Balakrishnan S, Arulmoorthy MP, Srinivasan M (2015) Production, purification and characterisation of extracellular L-asparaginase from salt marsh fungal endophytes. J Pharm Pharmac Sci 4:663–677
Selvaraj S, Murty VR (2017) Semi-solid state fermentation: A promising method for production and optimization of tannase from Bacillus gottheilii M2S2. Res J Biotechnol 12:39–48
Erva RR, Venkateswarulu TC, Pagala B (2018) 3 Biotech 8:24. https://doi.org/10.1007/s13205-017-1020-2
Ramakrishnan MSMS, Joseph R (1996) Characterization of an extracellular asparaginase of Rhodosporidiumtoruloides CBS14. Can J Microbiol 42:407–422. https://doi.org/10.1139/m96-047
Huang L, Liu Y, Sun Y, Yan Q, Jiang Z (2014) Biochemical characterization of a novel L-asparaginase with low glutaminase activity from Rhizomucor miehei and its application in food safety and leukemia treatment. Appl Environm Microbiol 80:1561–1569. https://doi.org/10.1128/AEM.03523-13
Lapmak K, Lumyong S, Thongkuntha S, Wongputtisin P, Sardsud U (2010) L-asparaginase production by Bipolaris sp BR438 isolated from brown rice in Thailand. Chiang Mai J Sci 37:160–164
Jalgaonwala RE, Mahajan RT (2014) Production of anticancer enzyme asparaginase from endophytic Eurotium sp. isolated from rhizomes of Curcuma longa. Euro J Exp Biol 4:36–43
Hassan SWM, Farag AM, Beltagy EA (2018) Purification, characterization and anticancer activity of L-asparaginase produced by marine Aspergillus terreus. J Pure Appl Microbiol 12:1845–1854. https://doi.org/10.22207/JPAM.12.4.19
Loureiro CB, Borges KS, Andrade AF, Tone LG, Said S (2012) Purification and biochemical characterization of native and pegylated form of l-asparaginase from Aspergillus terreus and evaluation of its antiproliferative activity. Adv Microbiol 2:138–145. https://doi.org/10.4236/aim.2012.22019
Funding
This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Grant No. 443953/2014–7) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) (Finance Code 001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Rosane Freitas Schwan
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ratuchne, A., Izidoro, S.C., Beitel, S.M. et al. A new extracellular glutaminase and urease-free l-asparaginase from Meyerozyma guilliermondii. Braz J Microbiol 54, 715–723 (2023). https://doi.org/10.1007/s42770-023-00939-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-00939-x