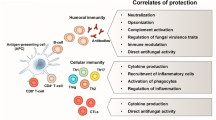
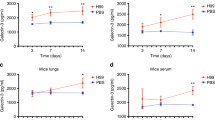
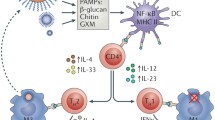
Abstract
Invasive fungal infections (IFI) are responsible for a large number of annual deaths. Most cases are closely related to patients in a state of immunosuppression, as is the case of patients undergoing chemotherapy. Cancer patients are severely affected by the worrisome proportions that an IFI can take during cancer progression, especially in an already immunologically and metabolically impaired patient. There is scarce knowledge about strategies to mitigate cancer progression in these cases, beyond conventional treatment with antifungal drugs with a narrow therapeutic range. However, in recent years, ample evidence has surfaced describing the possible interferences that IFI may have both on the progression of pre-existing cancers and in the induction of newly transformed cells. The leading gambit for modulation of tumor progression comes from the ability of fungal virulence factors to modulate the host’s immune system, since they are found in considerable concentrations in the tumor microenvironment during infection. In this context, cryptococcosis is of particular concern, since the main virulence factor of the pathogenic yeast is its polysaccharide capsule, which carries constituents with high immunomodulatory properties and cytotoxic potential. Therefore, we open a discussion on what has already been described regarding the progression of cryptococcosis in the context of cancer progression, and the possible implications that fungal glycan structures may take in both cancer development and progression.

Similar content being viewed by others
Data availability
Not applicable.
References
Diniz-Lima I, Fonseca LMd, Silva-Junior EBd, Guimarães-de-Oliveira JC, Freire-de-Lima L, Nascimento DO et al (2022) Cryptococcus: history, epidemiology and immune evasion. Appl Sci 12(14):7086. https://doi.org/10.3390/app12147086
Zaragoza O (2019) Basic principles of the virulence of Cryptococcus. Virulence 10(1):490–501. https://doi.org/10.1080/21505594.2019.1614383
Diniz-Lima I, da Fonseca LM, Dos Reis JS, da Costa Rodrigues, Santos MA, Costa KM, do Nascimento Santos CA et al (2022) The sweet side of fungal infections: structural glycan diversity and its importance for pathogenic adaptation. Med 9(6):37. https://doi.org/10.3390/medicines9060037
Steenbergen JN, Casadevall A (2003) The origin and maintenance of virulence for the human pathogenic fungus Cryptococcus neoformans. Microbes Infect 5(7):667–675. https://doi.org/10.1016/s1286-4579(03)00092-3
Bongomin F, Gago S, Oladele RO, Denning DW (2017) Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi 3(4):57. https://doi.org/10.3390/jof3040057
Strickland AB, Shi M (2021) Mechanisms of fungal dissemination. Cell Mol Life Sci 78(7):3219–3238. https://doi.org/10.1007/s00018-020-03736-z
Gow NA, Hube B (2012) Importance of the Candida albicans cell wall during commensalism and infection. Curr Opin Microbiol 15(4):406–412. https://doi.org/10.1016/j.mib.2012.04.005
Pagani JJ, Libshitz HI (1981) Opportunistic fungal pneumonias in cancer patients. AJR Am J Roentgenol 137(5):1033–1039. https://doi.org/10.2214/ajr.137.5.1033
Chaturvedi V, Chaturvedi S (2011) Cryptococcus gattii: a resurgent fungal pathogen. Trends Microbiol 19(11):564–571. https://doi.org/10.1016/j.tim.2011.07.010
Doering TL (2009) How sweet it is! Cell wall biogenesis and polysaccharide capsule formation in Cryptococcus neoformans. Annu Rev Microbiol 63:223–247. https://doi.org/10.1146/annurev.micro.62.081307.162753
Bach JF (2018) The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat Rev Immunol 18(2):105–120. https://doi.org/10.1038/nri.2017.111
Garn H, Potaczek DP, Pfefferle PI (2021) The hygiene hypothesis and new perspectives-current challenges meeting an old postulate. Front Immunol 12:637087. https://doi.org/10.3389/fimmu.2021.637087
Oikonomopoulou K, Brinc D, Kyriacou K, Diamandis EP (2013) Infection and cancer: revaluation of the hygiene hypothesis. Clin Cancer Res 19(11):2834–2841. https://doi.org/10.1158/1078-0432.CCR-12-3661
Christen U (2019) Pathogen infection and autoimmune disease. Clin Exp Immunol 195(1):10–14. https://doi.org/10.1111/cei.13239
Ezzati M, Pearson-Stuttard J, Bennett JE, Mathers CD (2018) Acting on non-communicable diseases in low- and middle-income tropical countries. Nature 559(7715):507–516. https://doi.org/10.1038/s41586-018-0306-9
Oh JK, Weiderpass E (2014) Infection and cancer: global distribution and burden of diseases. Ann Glob Health 80(5):384–392. https://doi.org/10.1016/j.aogh.2014.09.013
Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B (1975) An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA 72(9):3666–3670. https://doi.org/10.1073/pnas.72.9.3666
Lundin JI, Checkoway H (2009) Endotoxin and cancer. Environ Health Perspect 117(9):1344–1350. https://doi.org/10.1289/ehp.0800439
Krogh P, Hald B, Holmstrup P (1987) Possible mycological etiology of oral mucosal cancer: catalytic potential of infecting Candida albicans and other yeasts in production of N-nitrosobenzylmethylamine. Carcinogenesis 8(10):1543–1548. https://doi.org/10.1093/carcin/8.10.1543
Di Cosola M, Cazzolla AP, Charitos IA, Ballini A, Inchingolo F, Santacroce L (2021) Candida albicans and oral carcinogenesis A brief review. J Fungi 7(6):476. https://doi.org/10.3390/jof7060476
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140(6):883–899. https://doi.org/10.1016/j.cell.2010.01.025
Maeda M, Moro H, Ushijima T (2017) Mechanisms for the induction of gastric cancer by Helicobacter pylori infection: aberrant DNA methylation pathway. Gastric Cancer 20(Suppl 1):8–15. https://doi.org/10.1007/s10120-016-0650-0
Narunsky-Haziza L, Sepich-Poore GD, Livyatan I, Asraf O, Martino C, Nejman D et al (2022) Pan-cancer analyses reveal cancer-type-specific fungal ecologies and bacteriome interactions. Cell 185(20):3789–806 e17. https://doi.org/10.1016/j.cell.2022.09.005
Aykut B, Pushalkar S, Chen R, Li Q, Abengozar R, Kim JI et al (2019) The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 574(7777):264–267. https://doi.org/10.1038/s41586-019-1608-2
Skribek M, Rounis K, Afshar S, Grundberg O, Friesland S, Tsakonas G et al (2021) Effect of corticosteroids on the outcome of patients with advanced non-small cell lung cancer treated with immune-checkpoint inhibitors. Eur J Cancer 145:245–254. https://doi.org/10.1016/j.ejca.2020.12.012
Huet MAL, Lee CZ, Rahman S (2022) A review on association of fungi with the development and progression of carcinogenesis in the human body. Curr Res Microb Sci 3:100090. https://doi.org/10.1016/j.crmicr.2021.100090
Anderson PM, Lalla RV (2020) Glutamine for amelioration of radiation and chemotherapy associated mucositis during cancer therapy. Nutrients 12(6):1675. https://doi.org/10.3390/nu12061675
Lyman GH, Abella E, Pettengell R (2014) Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: a systematic review. Crit Rev Oncol Hematol 90(3):190–199. https://doi.org/10.1016/j.critrevonc.2013.12.006
Pagliano P, Esposito S, Ascione T, Spera AM (2020) Burden of fungal meningitis. Future Microbiol 15:469–472. https://doi.org/10.2217/fmb-2020-0006
Singh GK, Capoor MR, Nair D, Bhowmik KT (2017) Spectrum of fungal infection in head and neck cancer patients on chemoradiotherapy. J Egypt Natl Canc Inst 29(1):33–37. https://doi.org/10.1016/j.jnci.2017.01.006
Kyriakidis I, Tragiannidis A, Munchen S, Groll AH (2017) Clinical hepatotoxicity associated with antifungal agents. Expert Opin Drug Saf 16(2):149–165. https://doi.org/10.1080/14740338.2017.1270264
Tragiannidis A, Gkampeta A, Vousvouki M, Vasileiou E, Groll AH (2021) Antifungal agents and the kidney: pharmacokinetics, clinical nephrotoxicity, and interactions. Expert Opin Drug Saf 20(9):1061–1074. https://doi.org/10.1080/14740338.2021.1922667
Vincenzi B, Armento G, Spalato Ceruso M, Catania G, Leakos M, Santini D et al (2016) Drug-induced hepatotoxicity in cancer patients - implication for treatment. Expert Opin Drug Saf 15(9):1219–1238. https://doi.org/10.1080/14740338.2016.1194824
Perazella MA (2018) Pharmacology behind common drug nephrotoxicities. Clin J Am Soc Nephrol 13(12):1897–1908. https://doi.org/10.2215/CJN.00150118
Rausch CR, Kontoyiannis DP (2019) Prolonged voriconazole treatment in a patient with chronic lymphocytic leukemia resulting in a litany of chronic overlapping toxicities. J Oncol Pharm Pract 25(3):747–753. https://doi.org/10.1177/1078155218762624
Zimmerman LE, Rappaport H (1954) Occurrence of Cryptococcosis in patients with malignant disease of reticuloendothelial system. Am J Clin Pathol 24(9):1050–1072. https://doi.org/10.1093/ajcp/24.9.1050
Angarone M (2014) Fungal infections in cancer patients. Cancer Treat Res 161:129–155. https://doi.org/10.1007/978-3-319-04220-6_4
Ramirez-Garcia A, Rementeria A, Aguirre-Urizar JM, Moragues MD, Antoran A, Pellon A et al (2016) Candida albicans and cancer: can this yeast induce cancer development or progression? Crit Rev Microbiol 42(2):181–193. https://doi.org/10.3109/1040841X.2014.913004
Ramirez-Garcia A, Arteta B, Abad-Diaz-de-Cerio A, Pellon A, Antoran A, Marquez J et al (2013) Candida albicans increases tumor cell adhesion to endothelial cells in vitro: intraspecific differences and importance of the mannose receptor. PLoS One 8(1):e53584. https://doi.org/10.1371/journal.pone.0053584
Rodriguez-Cuesta J, Hernando FL, Mendoza L, Gallot N, de Cerio AA, Martinez-de-Tejada G et al (2010) Candida albicans enhances experimental hepatic melanoma metastasis. Clin Exp Metastasis 27(1):35–42. https://doi.org/10.1007/s10585-009-9300-9
Ramirez-Garcia A, Gallot N, Abad A, Mendoza L, Rementeria A, Hernando FL (2011) Molecular fractionation and characterization of a Candida albicans fraction that increases tumor cell adhesion to hepatic endothelium. Appl Microbiol Biotechnol 92(1):133–145. https://doi.org/10.1007/s00253-011-3540-8
Vadovics M, Ho J, Igaz N, Alfoldi R, Rakk D, Veres E, et al (2022) Candida albicans enhances the progression of oral squamous cell carcinoma in vitro and in vivo. mBio. e0314421. https://doi.org/10.1128/mBio.03144-21.
Kharadi U, Parkarwar P, Khairnar S, Arur P, Kulkarni T (2016) Oral candidiasis turns to oral cancer - a rare clinical presentation. Clin Oncol 1:1126
Alnuaimi AD, Ramdzan AN, Wiesenfeld D, O’Brien-Simpson NM, Kolev SD, Reynolds EC et al (2016) Candida virulence and ethanol-derived acetaldehyde production in oral cancer and non-cancer subjects. Oral Dis 22(8):805–814. https://doi.org/10.1111/odi.12565
Engku NasrullahSatiman EAF, Ahmad H, Ramzi AB, Abdul Wahab R, Kaderi MA, Wan Harun WHA et al (2020) The role of Candida albicans candidalysin ECE1 gene in oral carcinogenesis. J Oral Pathol Med 49(9):835–41. https://doi.org/10.1111/jop.13014
Buchanan KL, Murphy JW (1998) What makes Cryptococcus neoformans a pathogen? Emerg Infect Dis 4(1):71–83. https://doi.org/10.3201/eid0401.980109
Caira M, Trecarichi EM, Tumbarello M, Leone G, Pagano L (2011) Uncommon yeast infections in hematological patients: from diagnosis to treatment. Expert Rev Anti Infect Ther 9(11):1067–1075. https://doi.org/10.1586/eri.11.124
Paccoud O, Bougnoux ME, Desnos-Ollivier M, Varet B, Lortholary O, Lanternier F (2021) Cryptococcus gattii in patients with lymphoid neoplasms: an illustration of evolutive host-fungus interactions. J Fungi 7(3):212. https://doi.org/10.3390/jof7030212
Schmalzle SA, Buchwald UK, Gilliam BL, Riedel DJ (2016) Cryptococcus neoformans infection in malignancy. Mycoses 59(9):542–552. https://doi.org/10.1111/myc.12496
Almeida F, Wolf JM, da Silva TA, DeLeon-Rodriguez CM, Rezende CP, Pessoni AM et al (2017) Galectin-3 impacts Cryptococcus neoformans infection through direct antifungal effects. Nat Commun 8(1):1968. https://doi.org/10.1038/s41467-017-02126-7
LaRocque-de-Freitas IF, Rocha JDB, Nunes MP, Oliveira PAV, Nascimento DO, Freire-de-Lima L et al (2018) Involvement of the capsular GalXM-induced IL-17 cytokine in the control of Cryptococcus neoformans infection. Sci Rep 8(1):16378. https://doi.org/10.1038/s41598-018-34649-4
Rocha JD, Nascimento MT, Decote-Ricardo D, Corte-Real S, Morrot A, Heise N et al (2015) Capsular polysaccharides from Cryptococcus neoformans modulate production of neutrophil extracellular traps (NETs) by human neutrophils. Sci Rep 5:8008. https://doi.org/10.1038/srep08008
Villena SN, Pinheiro RO, Pinheiro CS, Nunes MP, Takiya CM, DosReis GA et al (2008) Capsular polysaccharides galactoxylomannan and glucuronoxylomannan from Cryptococcus neoformans induce macrophage apoptosis mediated by Fas ligand. Cell Microbiol 10(6):1274–1285. https://doi.org/10.1111/j.1462-5822.2008.01125.x
Albuquerque PC, Fonseca FL, Dutra FF, Bozza MT, Frases S, Casadevall A et al (2014) Cryptococcus neoformans glucuronoxylomannan fractions of different molecular masses are functionally distinct. Future Microbiol 9(2):147–161. https://doi.org/10.2217/fmb.13.163
Decote-Ricardo D, LaRocque-de-Freitas IF, Rocha JDB, Nascimento DO, Nunes MP, Morrot A et al (2019) Immunomodulatory role of capsular polysaccharides constituents of Cryptococcus neoformans. Front Med 6:129. https://doi.org/10.3389/fmed.2019.00129
Goldman DL, Lee SC, Casadevall A (1995) Tissue localization of Cryptococcus neoformans glucuronoxylomannan in the presence and absence of specific antibody. Infect Immun 63(9):3448–3453. https://doi.org/10.1128/iai.63.9.3448-3453.1995
Yamamoto S, Niki Y, Soejima R (1994) Fungal infection in hepatobiliary and pancreatic diseases: clinical evaluation in autopsy cases. Kansenshogaku Zasshi 68(5):612–616. https://doi.org/10.11150/kansenshogakuzasshi1970.68.612
Grinsell M, Weinhold LC, Cutler JE, Han Y, Kozel TR (2001) In vivo clearance of glucuronoxylomannan, the major capsular polysaccharide of Cryptococcus neoformans: a critical role for tissue macrophages. J Infect Dis 184(4):479–487. https://doi.org/10.1086/322787
Yao K, Qiu X, Hu H, Han Y, Zhang W, Xia R et al (2020) Pulmonary cryptococcosis coexisting with central type lung cancer in an immuocompetent patient: a case report and literature review. BMC Pulm Med 20(1):161. https://doi.org/10.1186/s12890-020-01200-z
Wei M, Xu YR, Liu K, Wen P (2020) Anastrozole-induced pulmonary cryptococcosis in a patient with early breast cancer: a case report. Medicine. 99(2):e18688. https://doi.org/10.1097/MD.0000000000018688
Ou KW, Hsu KF, Cheng YL, Hsu GC, Hsu HM, Yu JC (2010) Asymptomatic pulmonary nodules in a patient with early-stage breast cancer: Cryptococcus infection. Int J Infect Dis 14(1):e77-80. https://doi.org/10.1016/j.ijid.2009.03.007
Shah SI, Bui H, Velasco N, Rungta S (2017) Incidental finding of Cryptococcus on prostate biopsy for prostate adenocarcinoma following cardiac transplant: case report and review of the literature. Am J Case Rep 18:1171–1180. https://doi.org/10.12659/ajcr.905528
Wang X, Zhou Z, Turner D, Lilly M, Ou T, Jiang W (2022) Differential circulating fungal microbiome in prostate cancer patients compared to healthy control individuals. J Immunol Res 2022:2574964. https://doi.org/10.1155/2022/2574964
Williams SC, Sweeney J, Parameswaran L (2020) Diagnostic and management considerations in the modern patient with AIDS: a case of concurrent disseminated Kaposi sarcoma and colesional Cryptococcus neoformans. BMJ Case Rep 13(4):e233860. https://doi.org/10.1136/bcr-2019-233860
Silva LM, Ferreira WA, Filho R, Lacerda MVG, Ferreira GMA, Saunier MN et al (2020) New ST623 of Cryptococcus neoformans isolated from a patient with non-Hodgkin’s lymphoma in the Brazilian Amazon. Ann Clin Microbiol Antimicrob 19(1):20. https://doi.org/10.1186/s12941-020-00361-3
Dioverti MV, Parikh SA, Osmon DR, Habermann TM, Tande AJ (2019) Cryptococcus neoformans infections in patients with lymphoproliferative neoplasms. Leuk Lymphoma 60(4):920–926. https://doi.org/10.1080/10428194.2018.1508666
Averbuch D, Boekhoutt T, Falk R, Engelhard D, Shapiro M, Block C et al (2002) Fungemia in a cancer patient caused by fluconazole-resistant Cryptococcus laurentii. Med Mycol 40(5):479–484. https://doi.org/10.1080/mmy.40.5.479.484
Neves RP, Lima Neto RG, Leite MC, Silva VK, Santos Fde A, Macedo DP (2015) Cryptococcus laurentii fungaemia in a cervical cancer patient. Braz J Infect Dis 19(6):660–663. https://doi.org/10.1016/j.bjid.2015.06.014
Teoh F, Pavelka N (2016) How chemotherapy increases the risk of systemic candidiasis in cancer patients: current paradigm and future directions. Pathog 5(1):6. https://doi.org/10.3390/pathogens5010006
Infectious Diseases Working Party of the German Society of H, Oncology, Bohme A, Ruhnke M, Buchheidt D, Cornely OA, et al. Treatment of invasive fungal infections in cancer patients--recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann Hematol. 2009;88(2):97-110https://doi.org/10.1007/s00277-008-0622-5
Mota Fernandes C, Dasilva D, Haranahalli K, McCarthy JB, Mallamo J, Ojima I, et al. The future of antifungal drug therapy: novel compounds and targets. Antimicrob Agents Chemother. 2021;65(2). https://doi.org/10.1128/AAC.01719-20.
Brouwer AE, Rajanuwong A, Chierakul W, Griffin GE, Larsen RA, White NJ et al (2004) Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet 363(9423):1764–1767. https://doi.org/10.1016/S0140-6736(04)16301-0
McKeny PT, Nessel TA, Zito PM (2022) Antifungal antibiotics. StatPearls. Treasure Island (FL)
Cross JT Jr, Hickerson SL, Yamauchi T (1995) Antifungal drugs. Pediatr Rev 16(4):123–129. https://doi.org/10.1542/pir.16-4-123
van der Horst CM, Saag MS, Cloud GA, Hamill RJ, Graybill JR, Sobel JD et al (1997) Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. National Institute of Allergy and Infectious Diseases Mycoses Study Group and AIDS Clinical Trials Group. N Engl J Med 337(1):15–21. https://doi.org/10.1056/NEJM199707033370103
Pitisuttithum P, Negroni R, Graybill JR, Bustamante B, Pappas P, Chapman S et al (2005) Activity of posaconazole in the treatment of central nervous system fungal infections. J Antimicrob Chemother 56(4):745–755. https://doi.org/10.1093/jac/dki288
Saag MS, Graybill RJ, Larsen RA, Pappas PG, Perfect JR, Powderly WG et al (2000) Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin Infect Dis. 30(4):710–8. https://doi.org/10.1086/313757
Dvorak Z (2011) Drug-drug interactions by azole antifungals: beyond a dogma of CYP3A4 enzyme activity inhibition. Toxicol Lett 202(2):129–132. https://doi.org/10.1016/j.toxlet.2011.01.027
Lindsay J, Teh BW, Micklethwaite K, Slavin M (2019) Azole antifungals and new targeted therapies for hematological malignancy. Curr Opin Infect Dis 32(6):538–545. https://doi.org/10.1097/QCO.0000000000000611
Benitez LL, Carver PL (2019) Adverse effects associated with long-term administration of azole antifungal agents. Drugs 79(8):833–853. https://doi.org/10.1007/s40265-019-01127-8
van der Pas R, Hofland LJ, Hofland J, Taylor AE, Arlt W, Steenbergen J et al (2012) Fluconazole inhibits human adrenocortical steroidogenesis in vitro. J Endocrinol 215(3):403–412. https://doi.org/10.1530/JOE-12-0310
Albert SG, DeLeon MJ, Silverberg AB (2001) Possible association between high-dose fluconazole and adrenal insufficiency in critically ill patients. Crit Care Med 29(3):668–670. https://doi.org/10.1097/00003246-200103000-00039
Carvalho F, Louro F, Zakout R (2015) Adrenal insufficiency in metastatic lung cancer. World J Oncol 6(3):375–377. https://doi.org/10.14740/wjon890w
Wirk B (2011) Renal failure in multiple myeloma: a medical emergency. Bone Marrow Transplant 46(6):771–783. https://doi.org/10.1038/bmt.2011.8
Bancos I, Hazeldine J, Chortis V, Hampson P, Taylor AE, Lord JM et al (2017) Primary adrenal insufficiency is associated with impaired natural killer cell function: a potential link to increased mortality. Eur J Endocrinol 176(4):471–480. https://doi.org/10.1530/EJE-16-0969
Belvisi MG (2004) Regulation of inflammatory cell function by corticosteroids. Proc Am Thorac Soc 1(3):207–214. https://doi.org/10.1513/pats.200402-002MS
Obradovic MMS, Hamelin B, Manevski N, Couto JP, Sethi A, Coissieux MM et al (2019) Glucocorticoids promote breast cancer metastasis. Nature 567(7749):540–544. https://doi.org/10.1038/s41586-019-1019-4
Gompel A (2019) Hormone and breast cancer. Presse Med 48(10):1085–1091. https://doi.org/10.1016/j.lpm.2019.09.021
Starek-Swiechowicz B, Budziszewska B, Starek A (2021) Endogenous estrogens-breast cancer and chemoprevention. Pharmacol Rep 73(6):1497–1512. https://doi.org/10.1007/s43440-021-00317-0
Desai K, McManus JM, Sharifi N (2021) Hormonal therapy for prostate cancer. Endocr Rev 42(3):354–373. https://doi.org/10.1210/endrev/bnab002
Yassin A, AlRumaihi K, Alzubaidi R, Alkadhi S, Al AA (2019) Testosterone, testosterone therapy and prostate cancer. Aging Male 22(4):219–227. https://doi.org/10.1080/13685538.2018.1524456
Lenfant L, Leon P, Cancel-Tassin G, Audouin M, Staerman F, Roupret M et al (2020) Testosterone replacement therapy (TRT) and prostate cancer: an updated systematic review with a focus on previous or active localized prostate cancer. Urol Oncol 38(8):661–670. https://doi.org/10.1016/j.urolonc.2020.04.008
Williams K, Arron ST (2016) Association of CYP2C19 *17/*17 genotype with the risk of voriconazole-associated squamous cell carcinoma. JAMA Dermatol 152(6):719–720. https://doi.org/10.1001/jamadermatol.2016.0351
Mansh M, Binstock M, Williams K, Hafeez F, Kim J, Glidden D et al (2016) Voriconazole exposure and risk of cutaneous squamous cell carcinoma, Aspergillus colonization, invasive aspergillosis and death in lung transplant recipients. Am J Transplant 16(1):262–270. https://doi.org/10.1111/ajt.13431
Patel VA, Parikh SA, Nayyar PM, Ratner D (2015) Voriconazole-induced multiple squamous cell carcinomas in a patient with chronic lymphocytic leukemia. Dermatol Surg 41(6):747–749. https://doi.org/10.1097/DSS.0000000000000349
Epaulard O, Leccia MT, Blanche S, Chosidow O, Mamzer-Bruneel MF, Ravaud P et al (2011) Phototoxicity and photocarcinogenesis associated with voriconazole. Med Mal Infect 41(12):639–645. https://doi.org/10.1016/j.medmal.2011.09.016
Cheng MP, Paquette K, Lands LC, Ovetchkine P, Theoret Y, Quach C (2010) Voriconazole inhibition of vitamin A metabolism: are adverse events increased in cystic fibrosis patients? Pediatr Pulmonol 45(7):661–666. https://doi.org/10.1002/ppul.21234
Wang Q, He C (2020) Dietary vitamin A intake and the risk of ovarian cancer: a meta-analysis. Biosci Rep 40(4). https://doi.org/10.1042/BSR20193979
Bitsie KR, Cheng TD, McCann SE, Zirpoli G, Yao S, Bandera EV et al (2021) Dietary vitamin A and breast cancer risk in Black women: the African American Breast Cancer Epidemiology and Risk (AMBER) consortium. J Nutr 151(12):3725–3737. https://doi.org/10.1093/jn/nxab278
Zhang Z, Zhou L, Xie N, Nice EC, Zhang T, Cui Y et al (2020) Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct Target Ther 5(1):113. https://doi.org/10.1038/s41392-020-00213-8
Khachigian LM (2020) Repurposing drugs for skin cancer. Curr Med Chem 27(42):7214–7221. https://doi.org/10.2174/0929867327666191220103901
Weng N, Zhang Z, Tan Y, Zhang X, Wei X, Zhu Q (2022) Repurposing antifungal drugs for cancer therapy. J Adv Res. https://doi.org/10.1016/j.jare.2022.08.018
Chang HT, Chen WC, Chen JS, Lu YC, Hsu SS, Wang JL et al (2005) Effect of miconazole on intracellular Ca2+ levels and proliferation in human osteosarcoma cells. Life Sci 76(18):2091–2101. https://doi.org/10.1016/j.lfs.2004.09.033
Yang KC, Wu CC, Wu CH, Chen JH, Chu CH, Chen CH et al (2006) Involvement of proapoptotic Bcl-2 family members in terbinafine-induced mitochondrial dysfunction and apoptosis in HL60 cells. Food Chem Toxicol 44(2):214–226. https://doi.org/10.1016/j.fct.2005.07.008
Chen RJ, Lee WS, Liang YC, Lin JK, Wang YJ, Lin CH et al (2000) Ketoconazole induces G0/G1 arrest in human colorectal and hepatocellular carcinoma cell lines. Toxicol Appl Pharmacol 169(2):132–141. https://doi.org/10.1006/taap.2000.9062
Xu C, Zhuo Y, Liu Y, Chen H (2022) Itraconazole inhibits the growth of cutaneous squamous cell carcinoma by targeting HMGCS1/ACSL4 axis. Front Pharmacol 13:828983. https://doi.org/10.3389/fphar.2022.828983
Liang G, Liu M, Wang Q, Shen Y, Mei H, Li D et al (2017) Itraconazole exerts its anti-melanoma effect by suppressing Hedgehog, Wnt, and PI3K/mTOR signaling pathways. Oncotarget 8(17):28510–28525. https://doi.org/10.18632/oncotarget.15324
Liu R, Li J, Zhang T, Zou L, Chen Y, Wang K et al (2014) Itraconazole suppresses the growth of glioblastoma through induction of autophagy: involvement of abnormal cholesterol trafficking. Autophagy 10(7):1241–1255. https://doi.org/10.4161/auto.28912
Young DH, Wang NX, Meyer ST, Avila-Adame C (2018) Characterization of the mechanism of action of the fungicide fenpicoxamid and its metabolite UK-2A. Pest Manag Sci 74(2):489–498. https://doi.org/10.1002/ps.4743
Zhou Y, Xu J, Zhu Y, Duan Y, Zhou M (2016) Mechanism of action of the benzimidazole fungicide on Fusarium graminearum: interfering with polymerization of monomeric tubulin but not polymerized microtubule. Phytopathol 106(8):807–813. https://doi.org/10.1094/PHYTO-08-15-0186-R
Bae SH, Park JH, Choi HG, Kim H, Kim SH (2018) Imidazole antifungal drugs inhibit the cell proliferation and invasion of human breast cancer cells. Biomol Ther 26(5):494–502. https://doi.org/10.4062/biomolther.2018.042
Sarkar S, Singh PC (2019) Mechanistic aspects of fungicide-induced DNA damage: spectroscopic and molecular dynamics simulation studies. J Phys Chem B 123(41):8653–8661. https://doi.org/10.1021/acs.jpcb.9b06009
Li R, Liu B, Xu W, Yu L, Zhang C, Cheng J et al (2022) DNA damage and cell apoptosis induced by fungicide difenoconazole in mouse mononuclear macrophage RAW264.7. Environ Toxicol 37(3):650–9. https://doi.org/10.1002/tox.23432
Kim DJ, Kim J, Spaunhurst K, Montoya J, Khodosh R, Chandra K et al (2014) Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol 32(8):745–751. https://doi.org/10.1200/JCO.2013.49.9525
Shen PW, Chou YM, Li CL, Liao EC, Huang HS, Yin CH et al (2021) Itraconazole improves survival outcomes in patients with colon cancer by inducing autophagic cell death and inhibiting transketolase expression. Oncol Lett 22(5):768. https://doi.org/10.3892/ol.2021.13029
Zhang W, Zhou L, Qin S, Jiang J, Huang Z, Zhang Z et al (2021) Sertaconazole provokes proapoptotic autophagy via stabilizing TRADD in nonsmall cell lung cancer cells. MedComm 2(4):821–37. https://doi.org/10.1002/mco2.102
Chen Y, Chen HN, Wang K, Zhang L, Huang Z, Liu J et al (2019) Ketoconazole exacerbates mitophagy to induce apoptosis by downregulating cyclooxygenase-2 in hepatocellular carcinoma. J Hepatol 70(1):66–77. https://doi.org/10.1016/j.jhep.2018.09.022
Harris A (1997) Antiangiogenesis for cancer therapy. Lancet 349:13–15. https://doi.org/10.1016/s0140-6736(97)90014-3
Copley MS, Waldron M, Athans V, Welch SC, Brizendine KD, Cober E et al (2020) Itraconazole vs. posaconazole for antifungal prophylaxis in patients with acute myeloid leukemia undergoing intensive chemotherapy: a retrospective study. Int J Antimicrob Agents 55(3):105886. https://doi.org/10.1016/j.ijantimicag.2020.105886
Xin M, Ji X, De La Cruz LK, Thareja S, Wang B (2018) Strategies to target the Hedgehog signaling pathway for cancer therapy. Med Res Rev 38(3):870–913. https://doi.org/10.1002/med.21482
Tsubamoto H, Ueda T, Inoue K, Sakata K, Shibahara H, Sonoda T (2017) Repurposing itraconazole as an anticancer agent. Oncol Lett 14(2):1240–1246. https://doi.org/10.3892/ol.2017.6325
Lee J, Kang J, Kwon NY, Sivaraman A, Naik R, Jin SY et al (2021) Dual inhibition of P-gp and BCRP improves oral topotecan bioavailability in rodents. Pharm 13(4):559. https://doi.org/10.3390/pharmaceutics13040559
Locher KP (2016) Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat Struct Mol Biol 23(6):487–493. https://doi.org/10.1038/nsmb.3216
Beis K (2015) Structural basis for the mechanism of ABC transporters. Biochem Soc Trans 43(5):889–893. https://doi.org/10.1042/BST20150047
Prasad R, Balzi E, Banerjee A, Khandelwal NK (2019) All about CDR transporters: past, present, and future. Yeast 36(4):223–233. https://doi.org/10.1002/yea.3356
Prasad R, Murthy SK, Gupta V, Prasad R (1995) Multiple drug resistance in Candida albicans. Acta Biochim Pol 42(4):497–504. https://doi.org/10.18388/abp.1995_4902
Schuetzer-Muehlbauer M, Willinger B, Egner R, Ecker G, Kuchler K (2003) Reversal of antifungal resistance mediated by ABC efflux pumps from Candida albicans functionally expressed in yeast. Int J Antimicrob Agents 22(3):291–300. https://doi.org/10.1016/s0924-8579(03)00213-9
White TC, Marr KA, Bowden RA (1998) Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev 11(2):382–402. https://doi.org/10.1128/CMR.11.2.382
Kofla G, Turner V, Schulz B, Storch U, Froelich D, Rognon B et al (2011) Doxorubicin induces drug efflux pumps in Candida albicans. Med Mycol 49(2):132–142. https://doi.org/10.3109/13693786.2010.512022
Amawi H, Sim HM, Tiwari AK, Ambudkar SV, Shukla S (2019) ABC transporter-mediated multidrug-resistant cancer. Adv Exp Med Biol 1141:549–580. https://doi.org/10.1007/978-981-13-7647-4_12
Lu X, Wang Z, Huang H, Wang H (2020) Hedgehog signaling promotes multidrug resistance by regulation of ABC transporters in oral squamous cell carcinoma. J Oral Pathol Med 49(9):897–906. https://doi.org/10.1111/jop.13050
da Fonseca LM, da Silva VA, da Costa KM, Dos Reis JS, Previato JO, Previato LM et al (2022) Resistance to cisplatin in human lung adenocarcinoma cells: effects on the glycophenotype and epithelial to mesenchymal transition markers. Glycoconj J 39(2):247–259. https://doi.org/10.1007/s10719-022-10042-2
Wang T, Dong J, Yuan X, Wen H, Wu L, Liu J et al (2021) A new chalcone derivative C49 reverses doxorubicin resistance in MCF-7/DOX cells by inhibiting P-glycoprotein expression. Front Pharmacol 12:653306. https://doi.org/10.3389/fphar.2021.653306
Pruitt AA (1991) Central nervous system infections in cancer patients. Neurol Clin 9(4):867–888
Teixeira PA, Penha LL, Mendonca-Previato L, Previato JO (2014) Mannoprotein MP84 mediates the adhesion of Cryptococcus neoformans to epithelial lung cells. Front Cell Infect Microbiol 4:106. https://doi.org/10.3389/fcimb.2014.00106
Heung LJ, Hohl TM (2019) Inflammatory monocytes are detrimental to the host immune response during acute infection with Cryptococcus neoformans. PLoS Pathog 15(3):e1007627. https://doi.org/10.1371/journal.ppat.1007627
Qin HJ, Feng QM, Fang Y, Shen L (2014) Type-I interferon secretion in the acute phase promotes Cryptococcus neoformans infection-induced Th17 cell polarization in vitro. Exp Ther Med 7(4):869–872. https://doi.org/10.3892/etm.2014.1517
Angkasekwinai P, Sringkarin N, Supasorn O, Fungkrajai M, Wang YH, Chayakulkeeree M et al (2014) Cryptococcus gattii infection dampens Th1 and Th17 responses by attenuating dendritic cell function and pulmonary chemokine expression in the immunocompetent hosts. Infect Immun 82(9):3880–3890. https://doi.org/10.1128/IAI.01773-14
Kawakami K, Qureshi MH, Zhang T, Koguchi Y, Yara S, Takeda K et al (2000) Involvement of endogenously synthesized interleukin (IL)-18 in the protective effects of IL-12 against pulmonary infection with Cryptococcus neoformans in mice. FEMS Immunol Med Microbiol 27(3):191–200. https://doi.org/10.1111/j.1574-695X.2000.tb01430.x
Esmailbeig M, Ghaderi A (2017) Interleukin-18: a regulator of cancer and autoimmune diseases. Eur Cytokine Netw 28(4):127–140. https://doi.org/10.1684/ecn.2018.0401
Flaczyk A, Duerr CU, Shourian M, Lafferty EI, Fritz JH, Qureshi ST (2013) IL-33 signaling regulates innate and adaptive immunity to Cryptococcus neoformans. J Immunol 191(5):2503–2513. https://doi.org/10.4049/jimmunol.1300426
Diniz-Lima I, da Rosa PR, da Silva-Junior EB, Guimaraes-de-Oliveira JC, de Freitas EO, de Oliveira ND et al (2021) X-linked immunodeficient (XID) mice exhibit high susceptibility to Cryptococcus gattii infection. Sci Rep 11(1):18397. https://doi.org/10.1038/s41598-021-97041-9
Garcia-Barbazan I, Trevijano-Contador N, Rueda C, de Andres B, Perez-Tavarez R, Herrero-Fernandez I et al (2016) The formation of titan cells in Cryptococcus neoformans depends on the mouse strain and correlates with induction of Th2-type responses. Cell Microbiol 18(1):111–124. https://doi.org/10.1111/cmi.12488
Heyen L, Muller U, Siegemund S, Schulze B, Protschka M, Alber G et al (2016) Lung epithelium is the major source of IL-33 and is regulated by IL-33-dependent and IL-33-independent mechanisms in pulmonary cryptococcosis. Pathog Dis 74(7):ftw086. https://doi.org/10.1093/femspd/ftw086
Sun Z, Ji N, Ma Q, Zhu R, Chen Z, Wang Z et al (2020) Epithelial-mesenchymal transition in asthma airway remodeling is regulated by the IL-33/CD146 axis. Front Immunol 11:1598. https://doi.org/10.3389/fimmu.2020.01598
Taniguchi S, Elhance A, Van Duzer A, Kumar S, Leitenberger JJ, Oshimori N (2020) Tumor-initiating cells establish an IL-33-TGF-beta niche signaling loop to promote cancer progression. Science. 369(6501). https://doi.org/10.1126/science.aay1813.
Wiesner DL, Smith KD, Kotov DI, Nielsen JN, Bohjanen PR, Nielsen K (2016) Regulatory T cell induction and retention in the lungs drives suppression of detrimental type 2 Th cells during pulmonary cryptococcal infection. J Immunol 196(1):365–374. https://doi.org/10.4049/jimmunol.1501871
Eastman AJ, He X, Qiu Y, Davis MJ, Vedula P, Lyons DM et al (2015) Cryptococcal heat shock protein 70 homolog Ssa1 contributes to pulmonary expansion of Cryptococcus neoformans during the afferent phase of the immune response by promoting macrophage M2 polarization. J Immunol 194(12):5999–6010. https://doi.org/10.4049/jimmunol.1402719
Dong F, Ruan S, Wang J, Xia Y, Le K, Xiao X et al (2020) M2 macrophage-induced lncRNA PCAT6 facilitates tumorigenesis and angiogenesis of triple-negative breast cancer through modulation of VEGFR2. Cell Death Dis 11(9):728. https://doi.org/10.1038/s41419-020-02926-8
Tanaka A, Sakaguchi S (2017) Regulatory T cells in cancer immunotherapy. Cell Res 27(1):109–118. https://doi.org/10.1038/cr.2016.151
Supasorn O, Sringkarin N, Srimanote P, Angkasekwinai P (2016) Matrix metalloproteinases contribute to the regulation of chemokine expression and pulmonary inflammation in Cryptococcus infection. Clin Exp Immunol 183(3):431–440. https://doi.org/10.1111/cei.12725
Berois N, Pittini A, Osinaga E (2022) Targeting tumor glycans for cancer therapy: successes, limitations, and perspectives. Cancers 14(3):645. https://doi.org/10.3390/cancers14030645
Munkley J (2022) Aberrant sialylation in cancer: therapeutic opportunities. Cancers 14(17):4248. https://doi.org/10.3390/cancers14174248
Fernandes A, Azevedo CM, Silva MC, Faria G, Dantas CS, Vicente MM, et al. Glycans as shapers of tumour microenvironment: a sweet driver of T-cell-mediated anti-tumour immune response. Immunologyhttps://doi.org/10.1111/imm.13494
Vajaria BN, Patel PS (2017) Glycosylation: a hallmark of cancer? Glycoconj J 34(2):147–156. https://doi.org/10.1007/s10719-016-9755-2
Dos Reis JS, da Costa Rodrigues, Santos MA, Mendonca DP, do Martins Nascimento SI, Barcelos PM, de Correia Lima RG et al (2022) Glycobiology of cancer: sugar drives the show. Med 9(6):34. https://doi.org/10.3390/medicines9060034
dos Reis JS, Diniz-Lima I, Santos MARdC, Barcelos PM, da Costa KM, Valente RdC et al (2023) The blessed union of glycobiology and immunology: a marriage that worked. Med 10(2):15. https://doi.org/10.3390/medicines10020015
Rodrigues Mantuano N, Stanczak MA, Oliveira IA, Kirchhammer N, Filardy AA, Monaco G et al (2020) Hyperglycemia enhances cancer immune evasion by inducing alternative macrophage polarization through increased O-GlcNAcylation. Cancer Immunol Res 8(10):1262–1272. https://doi.org/10.1158/2326-6066.CIR-19-0904
Mantuano NR, Oliveira-Nunes MC, Alisson-Silva F, Dias WB, Todeschini AR (2019) Emerging role of glycosylation in the polarization of tumor-associated macrophages. Pharmacol Res. 146:104285. https://doi.org/10.1016/j.phrs.2019.104285
da Costa Santos MAR, Dos Reis JS, do Nascimento Santos CA, Costa KM, Barcelos PM, de Oliveira Francisco KQ et al (2023) Expression of O-glycosylated oncofetal fibronectin in alternatively activated human macrophages. Immunol Res 71(1):92–104. https://doi.org/10.1007/s12026-022-09321-9
Freire-de-Lima L, Gelfenbeyn K, Ding Y, Mandel U, Clausen H, Handa K et al (2011) Involvement of O-glycosylation defining oncofetal fibronectin in epithelial-mesenchymal transition process. Proc Natl Acad Sci USA 108(43):17690–17695. https://doi.org/10.1073/pnas.1115191108
Ding Y, Gelfenbeyn K, Freire-de-Lima L, Handa K, Hakomori SI (2012) Induction of epithelial-mesenchymal transition with O-glycosylated oncofetal fibronectin. FEBS Lett 586(13):1813–1820. https://doi.org/10.1016/j.febslet.2012.05.020
Alisson-Silva F, Freire-de-Lima L, Donadio JL, Lucena MC, Penha L, Sa-Diniz JN et al (2013) Increase of O-glycosylated oncofetal fibronectin in high glucose-induced epithelial-mesenchymal transition of cultured human epithelial cells. PLoS One 8(4):e60471. https://doi.org/10.1371/journal.pone.0060471
da Fonseca LM, Calvalhan DM, Previato JO, Mendonca Previato L, Freire-de-Lima L (2020) Resistance to paclitaxel induces glycophenotype changes and mesenchymal-to-epithelial transition activation in the human prostate cancer cell line PC-3. Tumour Biol 42(9):1010428320957506. https://doi.org/10.1177/1010428320957506
Freire-de-Lima L (2014) Sweet and sour: the impact of differential glycosylation in cancer cells undergoing epithelial-mesenchymal transition. Front Oncol 4:59. https://doi.org/10.3389/fonc.2014.00059
da Fonseca LM, da Silva VA, Freire-de-Lima L, Previato JO, Mendonca-Previato L, Capella MA (2016) Glycosylation in cancer: interplay between multidrug resistance and epithelial-to-mesenchymal transition? Front Oncol 6:158. https://doi.org/10.3389/fonc.2016.00158
Huang J, Lan C, Li H, Chen S, Lin Q, Weng H (2019) Concomitant lung adenocarcinoma and pulmonary cryptococcosis confirmed by pathologic examinations. Med 98(50):e18316. https://doi.org/10.1097/MD.0000000000018316
Ugrakli M, Araz M, Demirkiran A, Celik AF, Karakurt Eryilmaz M, Karaagac M et al (2022) Pneumonitis associated with trastuzumab emtansine in a patient with metastatic breast cancer. J Oncol Pharm Pract 28(3):740–745. https://doi.org/10.1177/10781552211066073
Ji YZ, Ma ZM, Chang L, Liu SR (2020) A novel case of cutaneous squamous cell carcinoma at the site of previous sporotrichosis. J Cosmet Dermatol 19(6):1487–1489. https://doi.org/10.1111/jocd.13158
Aslam S, Ghafoor S (2022) Association of Candida species with novel SARS-CoV-2 and biomarkers for fungal premalignant oral lesions. J Pak Med Assoc 72(9):1827–1830. https://doi.org/10.47391/JPMA.4632
Badheeb A, Al Gharem N, Al Hammadi S, Elsagheer S, Badheeb M, Ahmed F (2022) Primary pulmonary leiomyosarcoma with coexistent pulmonary aspergillosis: a case report. Pan Afr Med J 42:135. https://doi.org/10.11604/pamj.2022.42.135.34116
Scimeca M, Urbano N, Bonfiglio R, Duggento A, Toschi N, Schillaci O et al (2019) Novel insights into breast cancer progression and metastasis: a multidisciplinary opportunity to transition from biology to clinical oncology. Biochim Biophys Acta Rev Cancer 1872(1):138–148. https://doi.org/10.1016/j.bbcan.2019.07.002
Barco I, Garcia-Font M, Garcia-Fernandez A, Fraile M, Gimenez N, Gonzalez S et al (2021) Breast cancer patients developing distant metastasis at follow-up: mortality-related factors. Breast J 27(3):291–293. https://doi.org/10.1111/tbj.14159
Ward RA, Fawell S, Floc’h N, Flemington V, McKerrecher D, Smith PD (2021) Challenges and opportunities in cancer drug resistance. Chem Rev. 121(6):3297–351. https://doi.org/10.1021/acs.chemrev.0c00383
De Las RJ, Brozovic A, Izraely S, Casas-Pais A, Witz IP, Figueroa A (2021) Cancer drug resistance induced by EMT: novel therapeutic strategies. Arch Toxicol 95(7):2279–2297. https://doi.org/10.1007/s00204-021-03063-7
Li Y, Wang Z, Ajani JA, Song S (2021) Drug resistance and cancer stem cells. Cell Commun Signal 19(1):19. https://doi.org/10.1186/s12964-020-00627-5
Blackstock R, Casadevall A (1997) Presentation of cryptococcal capsular polysaccharide (GXM) on activated antigen-presenting cells inhibits the T-suppressor response and enhances delayed-type hypersensitivity and survival. Immunol 92(3):334–339. https://doi.org/10.1046/j.1365-2567.1997.00357.x
Lipovsky MM, Tsenova L, Coenjaerts FE, Kaplan G, Cherniak R, Hoepelman AI (2000) Cryptococcal glucuronoxylomannan delays translocation of leukocytes across the blood-brain barrier in an animal model of acute bacterial meningitis. J Neuroimmunol 111(1–2):10–14. https://doi.org/10.1016/s0165-5728(00)00354-4
Ellerbroek PM, Walenkamp AM, Hoepelman AI, Coenjaerts FE (2004) Effects of the capsular polysaccharides of Cryptococcus neoformans on phagocyte migration and inflammatory mediators. Curr Med Chem 11(2):253–266. https://doi.org/10.2174/0929867043456188
Ibrahim AS, Filler SG, Alcouloumre MS, Kozel TR, Edwards JE Jr, Ghannoum MA (1995) Adherence to and damage of endothelial cells by Cryptococcus neoformans in vitro: role of the capsule. Infect Immun 63(11):4368–4374. https://doi.org/10.1128/iai.63.11.4368-4374.1995
Chiapello LS, Baronetti JL, Aoki MP, Gea S, Rubinstein H, Masih DT (2004) Immunosuppression, interleukin-10 synthesis and apoptosis are induced in rats inoculated with Cryptococcus neoformans glucuronoxylomannan. Immunol 113(3):392–400. https://doi.org/10.1111/j.1365-2567.2004.01970.x
Chiapello LS, Aoki MP, Rubinstein HR, Masih DT (2003) Apoptosis induction by glucuronoxylomannan of Cryptococcus neoformans. Med Mycol 41(4):347–353. https://doi.org/10.1080/1369378031000137260
Retini C, Vecchiarelli A, Monari C, Bistoni F, Kozel TR (1998) Encapsulation of Cryptococcus neoformans with glucuronoxylomannan inhibits the antigen-presenting capacity of monocytes. Infect Immun 66(2):664–669. https://doi.org/10.1128/IAI.66.2.664-669.1998
Yauch LE, Lam JS, Levitz SM (2006) Direct inhibition of T-cell responses by the Cryptococcus capsular polysaccharide glucuronoxylomannan. PLoS Pathog. 2(11):e120. https://doi.org/10.1371/journal.ppat.0020120
Jain N, Li L, McFadden DC, Banarjee U, Wang X, Cook E et al (2006) Phenotypic switching in a Cryptococcus neoformans variety gattii strain is associated with changes in virulence and promotes dissemination to the central nervous system. Infect Immun 74(2):896–903. https://doi.org/10.1128/iai.74.2.896-903.2006
Jong A, Wu CH, Gonzales-Gomez I, Kwon-Chung KJ, Chang YC, Tseng HK et al (2012) Hyaluronic acid receptor CD44 deficiency is associated with decreased Cryptococcus neoformans brain infection. J Biol Chem 287(19):15298–15306. https://doi.org/10.1074/jbc.M112.353375
Chen C, Zhao S, Karnad A, Freeman JW (2018) The biology and role of CD44 in cancer progression: therapeutic implications. J Hematol Oncol 11(1):64. https://doi.org/10.1186/s13045-018-0605-5
Freire-de-Lima CG, Nascimento DO, Soares MB, Bozza PT, Castro-Faria-Neto HC, de Mello FG et al (2000) Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature 403(6766):199–203. https://doi.org/10.1038/35003208
Xiao YQ, Freire-de-Lima CG, Schiemann WP, Bratton DL, Vandivier RW, Henson PM (2008) Transcriptional and translational regulation of TGF-beta production in response to apoptotic cells. J Immunol 181(5):3575–3585. https://doi.org/10.4049/jimmunol.181.5.3575
Hao Y, Huang Y, Chen J, Li J, Yuan Y, Wang M et al (2020) Exopolysaccharide from Cryptococcus heimaeyensis S20 induces autophagic cell death in non-small cell lung cancer cells via ROS/p38 and ROS/ERK signalling. Cell Prolif 53(8):e12869. https://doi.org/10.1111/cpr.12869
Retini C, Kozel TR, Pietrella D, Monari C, Bistoni F, Vecchiarelli A (2001) Interdependency of interleukin-10 and interleukin-12 in regulation of T-cell differentiation and effector function of monocytes in response to stimulation with Cryptococcus neoformans. Infect Immun 69(10):6064–6073. https://doi.org/10.1128/IAI.69.10.6064-6073.2001
Funding
This work was supported by the Brazilian National Research Council (CNPq), the Brazilian Cancer Foundation, and the Rio de Janeiro State Science Foundation (FAPERJ).
Author information
Authors and Affiliations
Contributions
Conceptualization, Diniz-Lima I, da Fonseca LM, Freire-de-Lima CG, Freire-de-Lima L.
Technical support: Santos dos Reis J, Decote-Ricardo D, Morrot A.
Wrote the manuscript: Diniz-Lima I, da Fonseca LM, Freire-de-Lima L.
Figure elaboration: Diniz-Lima I.
Supervision: Previato JO, Mendonça Previato L, da Fonseca LM, Freire-de-Lima CG, Freire-de-Lima L.
Project administration: Freire-de-Lima L.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Fernando R. Pavan
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Diniz-Lima, I., da Fonseca, L.M., Dos Reis, J.S. et al. Non-self glycan structures as possible modulators of cancer progression: would polysaccharides from Cryptococcus spp. impact this phenomenon?. Braz J Microbiol 54, 907–919 (2023). https://doi.org/10.1007/s42770-023-00936-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-00936-0