Abstract
Monochoria hastata (L.) Solms (family Pontederiaceae), an ethnomedicinal aquatic herb, is used to remedy several gastrointestinal diseases by various ethnic groups in India. The present study aimed to purify and characterize the antibacterial active ingredient against gastrointestinal (GI) diseases and its mode of action using in vitro experimental models. The active lead molecule in the ethyl acetate extract (EA-Mh) fraction has been purified and characterized through high-performance liquid chromatography (HPLC), proton nuclear magnetic resonance (1H NMR), and electrospray ionization mass spectrometry (ESI–MS) methods. The anti-enteric efficacy has been evaluated against enteropathogenic Gram-positive and Gram-negative bacteria by minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), lactate dehydrogenase (LDH), and scanning electron microscopy (SEM) studies. The synergistic and antagonistic studies were done on E. coli MTCC 723 using standard antibiotics (ampicillin and kanamycin, final conc. 50 µg/ml) in a sterilized 96-well micro-plate, incubated at 37 ℃ for 24 h. The chromatographic and spectroscopic analyses revealed the presence of tridecanoic acid methyl ester (TAME) in the bioactive fraction. The compound causes significant extracellular leakage activity by disrupting cellular morphology in the Enterococcus faecalis MCC 2041 T and Salmonella enterica serovar Typhimurium MTCC 98, at a dose of 375 μg/ml and 750 μg/ml, respectively. The SEM study shows a significant rupturing of E. coli and E. faecalis cells due to TAME induced autolysis. It has synergistic activity with ampicillin. The in silico molecular docking through the AutoDock Vina 4.2 and GROMACS (ver. 5.1) Charmm27 force field results showed that the TAME had a strong binding affinity Escherichia coli DNA Gyrase B (PDB ID: 5l3j.pdb) protein and caused conformational changes. Thus, the manuscript reports the first time on the characterization of TAME from this plant with a detailed antibacterial mode of action studies.
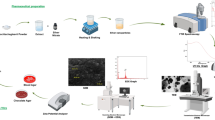
Graphical abstract





Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Newell DG, Koopmans M, Verhoef L, Duizer E, Aidara-Kane A, Sprong H, Opsteegh M, Langelaar M, Threfall J, Scheutz F, van der Giessen J (2010) Food-borne diseases—the challenges of 20 years ago still persist while new ones continue to emerge. Int J Food Microbiol 139:S3-15. https://doi.org/10.1016/j.ijfoodmicro.2010.01.021
Howard DH, Scott DR II, Packard R, Jones D (2003) Global impact of drug resistance. Clin Infect Dis 36(Suppl. 1):S4-10. https://doi.org/10.1086/344656
Friedman M (2015) Antibiotic-resistant bacteria: prevalence in food and inactivation by food-compatible compounds and plant extracts. J Agric Food Chem 63(15):3805–3822. https://doi.org/10.1021/acs.jafc.5b00778
Skov MN, Andersen JS, Aabo S, Ethelberg S, Aarestrup FM, Sørensen AH, ... Baggesen DL (2007) Antimicrobial drug resistance of Salmonella isolates from meat and humans, Denmark. Emerg Infect Dis 13(4):638. https://doi.org/10.3201/eid1304.060748.
Su LH, Chen HL, Liu SY, Chu C, Chia JH, Wu TL, Chiu CH (2006) Distribution of a transposon-like element carrying blaCMY-2 among Salmonella and other Enterobacteriaceae. J Antimicrob Chemother 57(3):424–429. https://doi.org/10.1093/jac/dki478
Yan JJ, Hong CY, Ko WC, Chen YJ, Tsai SH, Chuang CL, Wu JJ (2004) Dissemination of blaCMY-2 among Escherichia coli isolates from food animals, retail ground meats, and humans in southern Taiwan. Antimicrob Agents Chemother 48(4):1353–1356. https://doi.org/10.1128/AAC.48.4.1353-1356.2004
Pantosti A (2012) Methicillin-resistant Staphylococcus aureus associated with animals and its relevance to human health. Front Microbiol 3:127. https://doi.org/10.3389/fmicb.2012.00127
Lester CH, Frimodt-Møller N, Sørensen TL, Monnet DL, Hammerum AM (2006) In vivo transfer of the vanA resistance gene from an Enterococcus faecium isolate of animal origin to an E faecium isolate of human origin in the intestines of human volunteers. Antimicrob Agents Chemother 50(2):596–599. https://doi.org/10.1128/AAC.50.2.596-599.2006
Carlsson J (1984) Regulation of sugar metabolism in relation to the feast-and-famine existence of plaque. Cariology Today 205–11. Karger Publishers. https://doi.org/10.1159/000408740.
Lemos JA, Quivey RG Jr, Koo H, Abranches J (2013) Streptococcus mutans: a new Gram-positive paradigm? Microbiol 159(3):436. https://doi.org/10.1099/mic.0.066134-0
Tanwar J, Das S, Fatima Z, Hameed S (2014) Multidrug resistance: an emerging crisis. Interdisc Persp Infect Dis. https://doi.org/10.1155/2014/541340
Fair RJ, Tor Y (2014) Antibiotics and bacterial resistance in the 21st Century. Perspect Med Chem 6(6):25–64. https://doi.org/10.4137/PMC.S14459
Borris RP (1996) Natural products research: perspectives from a major pharmaceutical company. J Ethnopharm 51(1–3):29–38. https://doi.org/10.1016/0378-8741(95)01347-4
Cowan MM (1999) Plant products as antimicrobial agents. Clin Microb Rev 12(4):564–582. https://doi.org/10.1128/CMR.12.4.564
Tiwari S (2008) Plants: A rich source of herbal medicine. J Nat Prod 1:27–35
Lewis K, Ausubel FM (2006) Prospects for plant-derived antibacterials. Nat Biotech 24(12):1504–7. https://doi.org/10.1038/nbt1206-1504
Abdallah EM (2011) Plants: an alternative source for antimicrobials. J Appl PharmaSci 1(6):16–20
Kabara JJ, Swieczkowski DM, Conley AJ, Truant JP (1972) Fatty acids and derivatives as antimicrobial agents. Antimicrob Agents Chemothera 2(1):23–28. https://doi.org/10.1128/AAC.2.1.23
Kabara JJ (1984) Antimicrobial agents derived from fatty acids. J Am Oil Chemists’ Society 61(2):397–403. https://doi.org/10.1007/BF02678802
Kitahara T, Koyama N, Matsuda J, Aoyama Y, Hirakata Y, Kamihira S, Kohno S, Nakashima M, Sasaki H (2004) Antimicrobial activity of saturated fatty acids and fatty amines against methicillin-resistant Staphylococcus aureus. Biol Pharmaceut Bull 27(9):1321–1326. https://doi.org/10.1248/bpb.27.1321
P Desbois A (2012) Potential applications of antimicrobial fatty acids in medicine, agriculture and other industries. Recent Pat Antiinfective Drug Discov 7(2):111–22. https://doi.org/10.2174/157489112801619728
Freese E, Sheu C, Galliers E (1973) Function of lipophilic acids as antimicrobial food additives. Nature 241:321–325. https://doi.org/10.1038/241321a0
Chandrasekaran M, Kannathasan K, Venkatesalu V (2008) Antimicrobial activity of fatty acid methyl esters of some members of Chenopodiaceae. Zeitschrift für Naturforschung C 63(5–6):331–336. https://doi.org/10.1515/znc-2008-5-604
Chandrasekaran M, Senthilkumar A, Venkatesalu V (2011) Antibacterial and antifungal efficacy of fatty acid methyl esters from the leaves of Sesuvium portulacastrum L. Euro Rev Medical Pharmacol Sci 15(7):775–780
Venkatesalu V, Sundaramoorthy P, Anantharaj M, Gopalakrishnan M, Chandrasekaran M (2004) Studies on the fatty acid composition of marine algae of Rameswaram coast. Seaweed Res Util 26(1–2):83–86
McGaw LJ, Jäger AK, Van Staden J (2002) Antibacterial effects of fatty acids and related compounds from plants. S Afr J Bot 68(4):417–423. https://doi.org/10.1016/S0254-6299(15)30367-7
Misra D, Mandal M, Ghosh NN, Mandal V (2018) Pharmacognostic standardization of an ethnomedicinal aquatic herb, Monochoria hastata (L.) Solms for its antibacterial potentiality. Phcog J 10(3):533–40. https://doi.org/10.5530/pj.2018.3.87
Misra D, Mandal M, Ghosh NN, Mandal V (2020) Extraction and volatile compounds profiling of the bioactive fraction of Monochoria hastata (L.) Solms. Phcog Mag 16:S517-23. https://doi.org/10.4103/pm.pm_386_19
Misra D, Mandal M, Ghosh NN, Mandal V (2017) In vitro antioxidant and antibacterial activity and phytochemical profile of methanol extract of Monochoria hastata (L) Solms leaf. Int Res J Man Sc Tech 8(12):228–43. https://doi.org/10.32804/IRJMST
Chase MW, Christenhusz MJ, Fay MF, Byng JW, Judd WS, Soltis DE, Mabberley DJ, Sennikov AN, Soltis PS, Stevens PF (2016) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot J Linn Soc 181(1):1–20. https://doi.org/10.1111/boj.12385
Ajay KK, Lokanatha Rai KM, Umesha K (2002) Evaluation of antibacterial activity of 3, 5-dicyano-4, 6-diaryl-4-ethoxycarbonyl-piperid-2-ones. J Pharm Biomed Anal 27(5):837–840. https://doi.org/10.1016/s0731-7085(01)00456-3
Kumar G, Karthik L, Rao KB (2010) Antimicrobial activity of latex of Calotropis gigantea against pathogenic microorganisms-an in vitro study. Pharm online 3(3):155–163
Rios JL, Recio MC, Villar A (1991) Isolation and identification of the antibacterial compounds from Helichrysum stoechas. J Ethnopharm 33(1–2):51–55. https://doi.org/10.1016/0378-8741(91)90160-F
Mandal M, Paul S, Uddin MR, Mondal MA, Mandal S, Mandal V (2016) In vitro antibacterial potential of Hydrocotyle javanica Thunb. Asi Pac J Trop Dis 6(1):54–62. https://doi.org/10.1016/S2222-1808(15)60985-9
Hao G, Shi Y-H, Tang Y-L, Le G-W (2009) The membrane action mechanism of analogs of the antimicrobial peptide Buforin 2. Peptides 30(8):1421–1427. https://doi.org/10.1016/j.peptides.2009.05.016
Mandal V, Sen SK, Mandal NC (2010) Assessment of antibacterial activities of pediocin produced by Pediococcus acidilactici LAB 5. J Food Safe 30(3):635–651. https://doi.org/10.1111/j.1745-4565.2010.00230.x
Trott O, Olson AJ (2010) AutoDockVina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comp Chem 31(2):455–461. https://doi.org/10.1002/jcc.21334
Abraham MJ, van der Spoel D, Lindahl E, Hess B (2015) The GROMACS development team, GROMACS User Manual version 5.1. http://www.gromacs.org
Bjelkmar P, Larsson P, Cuendet MA, Hess B, Lindahl E (2010) Implementation of the CHARMM force field in GROMACS: analysis of protein stability effects from correction maps, virtual interaction sites, and water models. J Chem Theo Comp 6(2):459–466. https://doi.org/10.1021/ct900549r
Zoete V, Cuendet MA, Grosdidier A, Michielin O (2011) SwissParam: a fast force field generation tool for small organic molecules. J Comp Chem 32(11):2359–2368. https://doi.org/10.1002/jcc.21816
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera- a visualization system for exploratory research and analysis. J Comp Chem 25(13):1605–1612. https://doi.org/10.1002/jcc.20084
Guarrasi V, Mangione MR, Sanfratello V, Martorana V, Bulone D (2010) Quantification of underivatized fatty acids from vegetable oils by HPLC with UV detection. J Chromatogr Sci 48(8):663–668. https://doi.org/10.1093/chromsci/48.8.663
Hill IR, Levin IW (1979) Vibrational spectra and carbon-hydrogen stretching mode assignments for a series of n alkyl carboxylic acids. The J Chem Phys 70(2):842–851. https://doi.org/10.1063/1.437517
Dubey P, Sharma P, Kumar V (2017) FTIR and GC–MS spectral datasets of wax from Pinus roxburghii Sarg. needles biomass. Data in Brief 15:615–622. https://doi.org/10.1016/j.dib.2017.09.074
Mok HJ, Lee JW, Bandu R, Kang HS, Kim KH, Kim KP (2016) A rapid and sensitive profiling of free fatty acids using liquid chromatography-electrospray ionization tandem mass spectrometry (LC/ESI-MS/MS) after chemical derivatization. RSC Advan 6(38):32130–32139. https://doi.org/10.1039/C6RA01344A
Abad JL, Fabriás G, Camps FJTJ (2000) Synthesis of dideuterated and enantiomers of monodeuterated tridecanoic acids at C-9 and C-10 positions. J Organic Chem 65(25):8582–8588. https://doi.org/10.1021/jo000957k
Kerwin JL, Wiens AM, Ericsson LH (1996) Identification of fatty acids by electrospray mass spectrometry and tandem mass spectrometry. J Mass Spectro 31(2):184–192. https://doi.org/10.1002/(SICI)1096-9888(199602)31:2%3C184::AID-JMS283%3E3.0.CO;2-2
Chowdhury SK, Dutta T, Chattopadhyay AP, Ghosh NN, Chowdhury S, Mandal V (2021) Isolation of antimicrobial Tridecanoic acid from Bacillus sp. LBF-01 and its potentialization through silver nanoparticles synthesis: a combined experimental and theoretical studies. J Nanostruc Chem 20:1–5. https://doi.org/10.1007/s40097-020-00385-3
Belakhdar G, Benjouad A, Abdennebi EH (2015) Determination of some bioactive chemical constituents from Thesium humile Vahl. J Mater Environ Sci 6(10):2778–2783
Suresh A, Praveenkumar R, Thangaraj R, Oscar FL, Baldev E, Dhanasekaran D, Thajuddin N (2014) Microalgal fatty acid methyl ester a new source of bioactive compounds with antimicrobial activity. Asi Pac J Trop Dis 4:S979–S984. https://doi.org/10.1016/S2222-1808(14)60769-6
Vlietinck AJ, Van Hoof L, Totte J, Lasure A, Berghe DV, Rwangabo PC, Mvukiyumwami J (1995) Screening of hundred Rwandese medicinal plants for antimicrobial and antiviral properties. J Ethnopharmacol 46(1):31–47. https://doi.org/10.1016/0378-8741(95)01226-4
Parsons JB, Yao J, Frank MW, Jackson P, Rock CO (2012) Membrane disruption by antimicrobial fatty acids releases low-molecular-weight proteins from Staphylococcus aureus. J Bact 194(19):5294–5304. https://doi.org/10.1128/JB.00743-12
Desbois AP, Smith VJ (2010) Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol 85(6):1629–1642. https://doi.org/10.1007/s00253-009-2355-3
Yoon BK, Jackman JA, Valle-González ER, Cho N-J (2018) Antibacterial free fatty acids and monoglycerides: biological activities, experimental testing, and therapeutic applications. Int J Mol Sci 19(4):1114. https://doi.org/10.3390/ijms19041114
Galbraith H, Miller TB (1973) Physiological effects of long chain fatty acids on 10 bacterial cells and their protoplasts. J Appl Bacteriol 36:647–658. https://doi.org/10.1111/j.1365-2672.1973.tb04150.x
Sheu CW, Freese E (1972) Effects of fatty acids on growth and envelope proteins of Bacillus subtilis. J Bacteriol 111:516–524. https://doi.org/10.1128/jb.111.2.516-524.1972
Tsuchido T, Hiraoka T, Takano M, Shibasaki I (1985) Involvement of autolysin in cellular lysis of Bacillus subtilis induced by short- and medium-chain fatty acids. J Bacteriol 162:42–46. https://doi.org/10.1128/jb.162.1.42-46.1985
Tsuchido T, Ahn Y-H, Takano M (1987) Lysis of Bacillus subtilis cells by glycerol and sucrose esters of fatty acids. Appl Environ Microbiol 53:505–508. https://doi.org/10.1128/aem.53.3.505-508.1987
Tsuchido T, Kato Y, Ono K, Matsumura Y (1996) Killing of Bacillus subtilis by cell suicide through autolysis induction. Biocon Sci 1(1):19–24. https://doi.org/10.4265/bio.1.19
Bergsson G, Arnfinnsson J, SteingrÍmsson Ó, Thormar H (2001) Killing of Gram-positive cocci by fatty acids and monoglycerides. APMIS 109:670–678. https://doi.org/10.1034/j.1600-0463.2001.d01-131.x
Marr KA, Boeckh M, Carter RA, Kim HW, Corey L (2004) Combination antifungal therapy for invasive aspergillosis. Clin Infect Dis 39(6):797–802. https://doi.org/10.1086/423380
Rybak MJ, McGrath BJ (1996) Combination antimicrobial therapy for bacterial infections. Drugs 52(3):390–405. https://doi.org/10.2165/00003495-199652030-00005
Olajuyigbe OO, Afolayan AJ (2012) Synergistic interactions of methanolic extract of Acacia mearnsii De Wild. with antibiotics against bacteria of clinical relevance. Int J Mol Sci 13(7):8915–32. https://doi.org/10.3390/ijms13078915
Pei RS, Zhou F, Ji BP, Xu J (2009) Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. coli with an improved method. J Food Sci 74(7):M379-83. https://doi.org/10.1111/j.1750-3841.2009.01287.x
Delcour AH (2009) Outer membrane permeability and antibiotic resistance. Biochim Biophys Acta (BBA)-Proteins and Proteomics 1794(5):808–16. https://doi.org/10.1016/j.bbapap.2008.11.005
Chandrasekaran M, Venkatesalu V, Anantharaj M, Sivasankari S (2005) Antibacterial activity of fatty acid methyl esters of Ipomoea pes-caprae (L.) Sweet. Ind Dr 42:275–81
Özçelik B, Aslan M, Orhan I, Karaoglu T (2005) Antibacterial, antifungal, and antiviral activities of the lipophylic extracts of Pistacia vera. Microb Res 160(2):159–164. https://doi.org/10.1016/j.micres.2004.11.002
Walker JM (ed) (2002) The Protein Protocols Handbook: Second Edition, Humana Press Inc., 999 Riverview Drive, Suite 208, Totowa, New Jersey
Oussalah M, Caillet S, Lacroix M (2006) Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157: H7 and Listeria monocytogenes. J Food Prot 69(5):1046–1055. https://doi.org/10.4315/0362-028x-69.5.1046
Lambert RJW, Skandamis PN, Coote PJ, Nychas GJ (2001) A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microb 91(3):453–462. https://doi.org/10.1046/j.1365-2672.2001.01428.x
Churchward CP, Alany RG, Snyder LA (2018) Alternative antimicrobials: the properties of fatty acids and monoglycerides. Crit Rev Microbiol 44(5):561–570. https://doi.org/10.1080/1040841X.2018.1467875
Acknowledgements
The authors are thankful to KIIT, Bhubaneswar, the Chembiotek, Kolkata, and the Department of Chemistry, Visva-Bharati, Santiniketan, for their cooperation in ESI-MS and NMR analysis, respectively.
Author information
Authors and Affiliations
Contributions
Debabrata Misra: Conceptualization; data curation; formal analysis; investigation; methodology; validation; visualization; roles/writing—original draft.
Narendra Nath Ghosh: Software; validation; visualization; roles/writing—original draft.
Manab Mandal: Data curation; investigation; methodology.
Vivekananda Mandal: Data curation; investigation; methodology.
Nabajyoti Baildya: Software; visualization.
Sukhendu Mandal: Conceptualization; resources; validation; writing—review and editing.
Vivekananda Mandal: Conceptualization; formal analysis; funding acquisition; resources; supervision; validation; visualization; writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Fernando R. Pavan
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Misra, D., Ghosh, N.N., Mandal, M. et al. Anti-enteric efficacy and mode of action of tridecanoic acid methyl ester isolated from Monochoria hastata (L.) Solms leaf. Braz J Microbiol 53, 715–726 (2022). https://doi.org/10.1007/s42770-022-00696-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-022-00696-3