Abstract
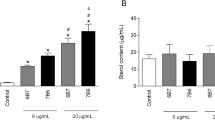
Corynebacterium pseudotuberculosis is a facultative intracellular pathogen that uses various mechanisms to survive within macrophages. In phagocytosis, this survival can be attributed to the ability to inhibit phagosome-lysosome fusion. In this fusion, some proteins, including Rabs GTPases, are involved in the maturation process and are responsible for regulating membrane vesicle trafficking. Thus, to better understand these mechanisms, the capacity of biofilm-producing and non biofilm-producing strains of Corynebacterium pseudotuberculosis for modulating the expression of endosomal proteins GTPases Rab 5 and Rab 7 was evaluated in an in vitro study of infection of goat macrophages. Blood was collected from ten Canindé goats, infected with biofilm-producing and non biofilm-producing strains of C. pseudotuberculosis. Blood cells were separated in colloidal silica-polyvinylpyrrolidone gradients (GE Healthcare®). These cells were maintained at 37 °C, with 5% of CO2. After differentiation, macrophages were infected with the mentioned strains. The bacterial pellets were marked with Rab 5 and Rab 7 antibodies, and their expression was observed by flow cytometry. Both strains of C. pseudotuberculosis (biofilm-producing and non biofilm-producing) were observed to be capable of altering the expression of Rab proteins in macrophages cultivated in vitro. Macrophages from the animals infected with the biofilm-producing strain had an increase in the expression of Rab 5 protein, mainly when these macrophages were treated with the non biofilm-producing strain. The same mechanism was shown to function with Rab 7 protein, however at a lower intensity of expression when compared with Rab 5.




Similar content being viewed by others
Data availability
All data and material are available under request.
Code availability
Not applicable.
References
Vieira OV, Botelho RJ, Grinstein S (2002) Phagosome maturation: aging gracefully. Biochem J 366(3):689–7004. https://doi.org/10.1042/BJ20020691
Hutagalung AH, Novick PJ (2011) Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 91(1):119–149. https://doi.org/10.1152/physrev.00059.2009
Szatmári Z, Sass M (2014) The autophagic roles of Rab small GTPases and their upstream regulators: a review. Autophagy 10(7):1154–1166. https://doi.org/10.4161/auto.29395
Bogdan JR, Newlands-Monteith CF, Ellis JA (1997) Nitric oxide production following in vitro stimulation of ovine pulmonary alveolar macrophages. Vet Immunol Immunopathol 56(3–4):299–310. https://doi.org/10.1016/S0165-2427(96)05748-0
Hard GC (1975) Comparative toxic effect of the surface lipid of Corynebacterium ovis on peritoneal macrophages. Infect Immun 12(6):1439–1449
Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Jappin-Scott HM (1995) Microbial biofilms. Annu Rev Microbiol 49(1):711–745
Mah T, O’toole GA (2001) Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9(1):34–39. https://doi.org/10.1016/s0966-842x(00)01913-2
Olson ME, Ceri H, Morck DW, Buret AG, Read RR (2002) Biofilm bacteria: formation and comparative susceptibility to antibiotics. Can J Vet Res 66(2):86–92
Costerton JW, Stewart PS, Greeberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284(5418):318–322. 101126/science.284.5418.1318
Morato CI, Silva IA Jr, Borges AF, Dorta ML, Oliveira MAP, Jancar S, Serezani CH, Dias FR (2014) Essential role of leukotriene B4 on Leishmania (Viannia) braziliensis killing by human macrophages. Microbes Infect 16(11):945–953. https://doi.org/10.1016/j.micinf.2014.08015
Sampaio PS, Vale VLC, Costa LFM, Fraga, RE, Santos, HHM, Sá MCA, Bastos BL, Rocha Filho JTR, Trindade SC, Nascimento RJM (2019)Padronização de técnicas por citometria de fluxo, para avaliar Corynebacterium pseudotuberculosis e células fagocitárias murinas. Pubvet 13:(11)1–9. https://doi.org/10.31533/pubvet.v13n11a443.1-9
Frati RA, Backer JM, Gruenberg J, Corvera S, Deretic V (2011) Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J Cell Biol 154(3):631–644. https://doi.org/10.1083/jcb.200106049
Ory S, Gasman S (2011) Rho GTPases and exocytosis: what are the molecular links? In:Seminars in cell & developmental biology. Academic Press 22(1):27–32. https://doi.org/10.1016/j.semcdb.2010.12.002
Armentia MML, Amaya C, Colombo MI (2016) Rab GTPases and the autophagy pathway: bacterial targets for a suitable biogenesis and trafficking of their own vacuoles. Cells 5(1):11. https://doi.org/10.3390/cells5010011
Jordens I, Fernandez-Borja M, Marsman M, Dusseljee S, Janssen L, Calafat J, Janssen H, Wubbolts R, Neefies J (2001) The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr Biol 11(21):1680–1685. https://doi.org/10.1016/s0960-9822(01)00531-0
Sun J, Deghmane AE, Soualhine H, Hong T, Bucci C, Solodkin A, Hmama S (2007) Mycobacterium bovis BCG disrupts the interaction of Rab7 with RILP contributing to inhibition of phagosome maturation. J Leukoc Biol 82(6):1437–1445. https://doi.org/10.1189/jlb.10.1189
Johansson M, Rocha N, Zwart W, Jordens I, Janssen L, Kuijl C, Olkkonen VM, Neefjes J (2007) Activation of endosomal dynein motors by stepwise assembly of Rab7–RILP–p150Glued, ORP1L, and the receptor βlll spectrin. J Cell Biol 176(4):459–471. https://doi.org/10.1083/jcb.200606077
Flannagan RS, Cosio G, Grinstein S (2009) Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol 7(5):355–366. https://doi.org/10.1038/nrmicro2128
Poteryaev D, Datta S, Ackema K, Zerial M, Spang A (2010) Identification of the switch in early-to-late endosome transition. Cell 14(3):497–508. https://doi.org/10.1016/j.cell.2010.03011
Souza AP (2014) Avaliação in vitro de aspectos da resposta imune à Corynebacterium pseudotuberculosis atenuada e selvagem. Tese, Universidade Federal da Bahia
Acknowledgements
The authors are grateful to FAPEX (Fundação de Apoio á Pesquisa e a Extenção, UFBA – Universidade Federal da Bahia) for the financial support for the project and FAPESB (Fundação de Amparo á Pesquisa do Estado da Bahia) for granting the scholarship to the first author.
Funding
This work was funded by the Fundação de Apoio à Pesquisa do Estado da Bahia (FAPESB) grant number Nº BOL0275/2014.
Author information
Authors and Affiliations
Contributions
MCSA, MMC, and RM designed the project and discussed the results of the experiments. MCSA, MEC, MCS, and JTRRF developed methodologies, and MMS and ASS performed flow cytometry. MCSA, MMC, and RM prepared the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This experiment was authorized by Ethics Commission on the Use of Animals of the Institute of Health Sciences (CEUA—ICS) protocol number 123/2017.
Consent to participate
Not applicable.
Consent for publication
All authors consent for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Mariana X Byndloss
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Conceição Aquino de Sá, M., Filho, J.T.R.R., Alcantara, M.E. et al. Analysis of Gtpases Rab 5 and Rab 7 expression from macrophages infected with biofilm-producing and non biofilm-producing strains of Corynebacterium pseudotuberculosis. Braz J Microbiol 53, 447–453 (2022). https://doi.org/10.1007/s42770-021-00665-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00665-2