Abstract
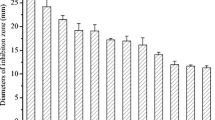
Actinomycetes due to their unique repertoire of antimicrobial secondary metabolites can be an eco-friendly and sustainable alternative to agrochemicals to control plant pathogens. In the present study, antifungal activity of twenty different actinomycetes was evaluated via dual culture plate assay against six different phytopathogens, viz., Alternaria alternata, Aspergillus flavus, Fusarium oxysporum f. sp. lycopersici, Sarocladium oryzae, Sclerotinia sclerotiorum, and Rhizoctonia solani. Two potential isolates, Streptomyces amritsarensis V31 and Kribella karoonensis MSCA185 showing high antifungal activity against all six fungal pathogens, were further evaluated after extraction of bioactive metabolites in different solvents. Metabolite extracted from S. amritsarensis V31 in different solvents inhibited Rhizoctonia solani (7.5–65%), Alternaria alternata (5.5–52.7%), Aspergillus flavus (8–30.7%), Fusarium oxysporum f. sp. lycopersici (25–44%), Sarocladium oryzae (11–55.5%), and Sclerotinia sclerotiorum (29.7–40.5%); 1000 D diluted methanolic extract of S. amritsarensis V31 showed growth inhibition against R. solani (23.3%), A. flavus (7.7%), F. oxysporum (22.2%), S. oryzae (16.7%), and S. sclerotiorum (19.0%). Metabolite extracts of S. amritsarensis V31 significantly reduced the incidence of rice sheath blight both as preventive and curative sprays. Chemical profiling of the metabolites in DMSO extract of S. amritsarensis V31 revealed 6-amino-5-nitrosopyrimidine-2,4-diol as the predominant compound present. It was evident from the LC–MS analyses that S. amritsarensis V31 produced a mixture of potential antifungal compounds which inhibited the growth of different phytopathogenic fungi. The results of this study indicated that metabolite extracts of S. amritsarensis V31 can be exploited as a bio-fungicide to control phytopathogenic fungi.








Similar content being viewed by others
Data availability
Not applicable.
References
Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ (2012) Emerging fungal threats to animal, plant and ecosystem health. Nature 484(7393):186–194. https://doi.org/10.1038/nature10947
Garcia-Solache MA, Casadevall A (2010) Global warming will bring new fungal diseases for mammals. mBio 1(1):e00061-10. https://doi.org/10.1128/mBio.00061-10
Scarpino V, Reyneri A, Sulyok M, Krska R, Blandino M (2015) Effect of fungicide application to control Fusarium head blight and 20 Fusarium and Alternaria mycotoxins in winter wheat (Triticum aestivum L.). World Mycotoxin J 8(4):499–510. https://doi.org/10.3920/WMJ2014.1814
Talabi AO, Kayode TJ (2019) Groundwater pollution and remediation. J Water Resour Prot 11(1):1–9. https://doi.org/10.4236/jwarp.2019.111001
Elahi E, Weijun C, Zhang H, Nazeer M (2019) Agricultural intensification and damages to human health in relation to agrochemicals: application of artificial intelligence. Land Use Pol 83:461–474. https://doi.org/10.1016/j.landusepol.2019.02.023
Mohite OS, Weber T, Kim HU, Lee SY (2019) Genome scale metabolic reconstruction of actinomycetes for antibiotics production. Biotechnol J 14(1):1800377. https://doi.org/10.1002/biot.201800377
Savi DC, Shaaban KA, Gos FM, Thorson JS, Glienke C, Rohr J (2019) Secondary metabolites produced by Microbacterium sp. LGMB471 with antifungal activity against the phytopathogen Phyllosticta citricarpa. Folia Microbiol 64(3):453–60. https://doi.org/10.1007/s12223-018-00668-x
Kumar M, Kumar P, Das P, Kapur MK (2019) Draft genome of Streptomyces sp. strain 130 and functional analysis of extracellular enzyme producing genes. Mol Biol Rep 46(5):5063–5071. https://doi.org/10.1007/s11033-019-04960-y
Verma VC, Singh SK, Prakash S (2011) Bio-control and plant growth promotion potential of siderophore producing endophytic Streptomyces from Azadirachta indica A. Juss J Basic Microbiol 51(5):550–556. https://doi.org/10.1002/jobm.201000155
Anwar S, Ali B, Sajid I (2016) Screening of rhizospheric actinomycetes for various in-vitro and in-vivo plant growth promoting (PGP) traits and for agroactive compounds. Front Microbiol 7:1334. https://doi.org/10.3389/fmicb.2016.01334
Palakawong NAS, Pristaš P, Hrehová L, Javorský P, Stams AJ, Plugge CM (2016) Actinomyces succiniciruminis sp. nov. and Actinomyces glycerinitolerans sp. nov., two novel organic acid-producing bacteria isolated from rumen. Syst Appl Microbiol 39(2):445–452. https://doi.org/10.1016/j.syapm.2016.08.001
Palla MS, Guntuku GS, Muthyala MKK, Pingali S, Sahu PK (2018) Isolation and molecular characterization of antifungal metabolite producing actinomycete from mangrove soil. Beni-Suef Univ J Basic Appl Sci 7(2):250–256. https://doi.org/10.1016/j.bjbas.2018.02.006
Shi L, Nwet TT, Ge B, Zhao W, Liu B, Cui H, Zhang K (2018) Antifungal and plant growth-promoting activities of Streptomyces roseoflavus strain NKZ-259. Biol Cont 125:57–64. https://doi.org/10.1016/j.biocontrol.2018.06.012
Jakubiec-Krzesniak K, Rajnisz-Mateusiak A, Guspiel A, Ziemska J, Solecka J (2018) Secondary metabolites of actinomycetes and their antibacterial, antifungal and antiviral properties. Pol J Microbiol 67(3):259–272. https://doi.org/10.21307/pjm-2018-048
Ahmad MS, El-Gendy AO, Ahmed RR, Hassan HM, El-Kabbany HM, Merdash AG (2017) Exploring the antimicrobial and antitumor potentials of Streptomyces sp. AGM12–1 isolated from Egyptian soil. Front Microbiol 8:438. https://doi.org/10.3389/fmicb.2017.00438
Han L, Zhang G, Miao G, Zhang X, Feng J (2015) Streptomyces kanasensis sp. nov., an antiviral glycoprotein producing actinomycete isolated from forest soil around Kanas lake of China. Curr Microbiol 71(6):627–631. https://doi.org/10.1007/s00284-015-0900-0
Ruanpanun P, Laatsch H, Tangchitsomkid N, Lumyong S (2011) Nematicidal activity of fervenulin isolated from a nematicidal actinomycete, Streptomyces sp. CMU-MH021, on Meloidogyne incognita. World J Microbiol Biotechnol 27(6):1373–80. https://doi.org/10.1007/s11274-010-0588-z
Qi D, Zou L, Zhou D, Chen Y, Gao Z, Feng R, Zhang M, Li K, Xie J, Wang W (2019) Taxonomy and broad-spectrum antifungal activity of Streptomyces sp. SCA3–4 isolated from rhizosphere soil of Opuntia stricta. Front Microbiol 10:1390. https://doi.org/10.3389/fmicb.2019.01390
Zhao S, Liu C, Zheng W, Ma Z, Cao T, Zhao J, Yan K, Xiang W, Wang X (2017) Micromonospora parathelypteridis sp. nov., an endophytic actinomycete with antifungal activity isolated from the root of Parathelypteris beddomei (Bak.) Ching. Int J Syst Evol Microbiol 67(2):268–274. https://doi.org/10.1099/ijsem.0.001614
Orozco-Mosqueda CM, Valencia-Cantero E, Lopez-Albarran P, Martinez-Pacheco M, Velazquez-Becerra C (2015) Bacterium Arthrobacter agilis UMCV2 and diverse amines inhibit in vitro growth of wood-decay fungi. Rev Argent Microbiol 47(3):219–228. https://doi.org/10.1016/j.ram.2015.06.005
Keel C, Weller DM, Natsch A, Defago G, Cook RJ, Thomashow LS (1996) Conservation of the 2,4-diacetylphloroglucinol biosynthesis locus among fluorescent Pseudomonas strains from diverse geographic locations. Appl Environ Microbiol 62(2):552–563. https://doi.org/10.1128/AEM.62.2.552-563.1996
Mahdizadeh V, Safaie N, Khelghatibana F (2015) Evaluation of antifungal activity of silver nanoparticles against some phytopathogenic fungi and Trichoderma harzianum. J Crop Prot 4(3):291–300
Liu CX, Zhang J, Wang XJ, Qian PT, Wang JD, Gao YM, Yan YJ, Zhang SZ, Xu PF, Li WB, Xiang WS (2012) Antifungal activity of borrelidin produced by a Streptomyces strain isolated from soybean. J Agric Food Chem 60(5):1251–1257. https://doi.org/10.1021/jf2044982
Tepe B, Daferera D, Sokmen A, Sokmen M, Polissiou M (2005) Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem 90(3):333–340. https://doi.org/10.1016/j.foodchem.2003.09.013
Sharma NR, Teng PS, Oliver FM (1990) Comparisons of assessment methods for rice sheath blight disease. Philipp Phytopathol 26(12):20–24
Almeida F, Rodrigues ML, Coelho C (2019) The still underestimated problem of fungal diseases worldwide. Front Microbiol 10:214. https://doi.org/10.3389/fmicb.2019.00214
Derbyshire MC, Denton-Giles M (2016) The control of Sclerotinia stem rot on oilseed rape (Brassica napus): current practices and future opportunities. Plant Pathol 65(6):859–877. https://doi.org/10.1111/ppa.12517
Moni ZR, Ali MA, Alam MS, Rahman MA, Bhuiyan MR, Mian MS, Iftekharuddaula MK, Latif MA, Khan MAI (2016) Morphological and genetical variability among Rhizoctonia solani isolates causing sheath blight disease of rice. Rice Sci 23(1):42–50. https://doi.org/10.1016/j.rsci.2016.01.005
Guimarães RA, Lobo VL, Côrtes MV, Filippi MC, Prabhu AS (2017) Characterization of Sarocladium oryzae and its reduction potential of rice leaf blast. Pesqui Agropecu Trop 47(1):41–52. https://doi.org/10.1590/1983-40632016v4742738
Ramírez-Cariño HF, Guadarrama-Mendoza PC, Sánchez-López V, Cuervo-Parra JA, Ramírez-Reyes T, Dunlap CA, Valadez-Blanco R (2020) Biocontrol of Alternaria alternata and Fusarium oxysporum by Trichoderma asperelloides and Bacillus paralicheniformis in tomato plants. Antonie Van Leeuwenhoek 113(9):1247–1261. https://doi.org/10.1007/s10482-020-01433-2
Baibakova EV, Nefedjeva EE, Suska-Malawska M, Wilk M, Sevriukova GA, Zheltobriukhov VF (2019) Modern fungicides: mechanisms of action, fungal resistance and phytotoxic effects. Annu Res Rev Biol 32(3):1–16. https://doi.org/10.9734/arrb/2019/v32i330083
Rani L, Thapa K, Kanojia N, Sharma N, Singh S, Grewal AS, Srivastav AL, Kaushal J (2020) An extensive review on the consequences of chemical pesticides on human health and environment. J Cleaner Prod 283:124657. https://doi.org/10.1016/j.jclepro.2020.124657
Ding T, Yang LJ, Zhang WD, Shen YH (2019) The secondary metabolites of rare actinomycetes: chemistry and bioactivity. RSC Adv 9(38):21964–21988. https://doi.org/10.1039/C9RA03579F
Zhang D, Lu Y, Chen H, Wu C, Zhang H, Chen L, Chen X (2020) Antifungal peptides produced by actinomycetes and their biological activities against plant diseases. J Antibiot 73(5):265–282. https://doi.org/10.1038/s41429-020-0287-4
Weber T, Charusanti P, Musiol-Kroll EM, Jiang X, Tong Y, Kim HU, Lee SY (2015) Metabolic engineering of antibiotic factories: new tools for antibiotic production in actinomycetes. Trends Biotechnol 33(1):15–26. https://doi.org/10.1016/j.tibtech.2014.10.009
Tian SZ, Pu X, Luo G, Zhao LX, Xu LH, Li WJ, Luo Y (2013) Isolation and characterization of new p-terphenyls with antifungal, antibacterial, and antioxidant activities from halophilic actinomycete Nocardiopsis gilva YIM 90087. J Agric Food Chem 61(12):3006–3012. https://doi.org/10.1021/jf400718w
Sinha K, Hegde R, Kush A (2014) Exploration on native actinomycetes strains and their potential against fungal plant pathogens. Int J Curr Microbiol App Sci 3(11):37–45
Fang X, Shen J, Wang J, Chen ZL, Chen ZY, Liu LY, Zeng HX, Jin XB (2018) Antifungal activity of 3-acetylbenzamide produced by actinomycete WA23-4-4 from the intestinal tract of Periplaneta americana. J Microbiol 56:516–523. https://doi.org/10.1007/s12275-018-7510-z
Baz M, Lahbabi D, Samri S, Val F, Hamelin G, Madore I, Bouarab K, Beaulieu C, Ennaji MM, Barakate M (2012) Control of potato soft rot caused by Pectobacterium carotovorum and Pectobacterium atrosepticum by Moroccan actinobacteria isolates. World J Microbiol Biotechnol 28:303–311. https://doi.org/10.1007/s11274-011-0820-5
Do E, Lee HG, Park M, Cho YJ, Kim DH, Park SH, Eun D, Park T, An S, Jung WH (2019) Antifungal mechanism of action of lauryl betaine against skin-associated fungus Malassezia restricta. Mycobiol 47(2):242–249. https://doi.org/10.1080/12298093.2019.1625175
Ivanova V, Lyutskanova D, Kolarova M, Aleksieva K, Raykovska V, Stoilova-Disheva M (2010) Structural elucidation of a bioactive metabolites produced by Streptomyces avidinii SB9 strain, isolated from permafrost soil in Spitsbergen. Arctic Biotechnol Biotechnol Equip 24(4):2092–2095. https://doi.org/10.2478/V10133-010-0080-9
Abd El-Wahab AHF (2012) Synthesis, reactions and evaluation of the antimicrobial activity of some 4-(p-Halophenyl)-4H-naphthopyran, pyranopyrimidine and pyranotriazolopyrimidine derivatives. Pharm 5(7):745–757. https://doi.org/10.3390/ph5070745
Olivella M, Marchal A, Nogueras M, Melguizo M, Lima B et al (2015) New series of antibacterial nitrosopyrimidines: synthesis and structure–activity relationship. Arch Pharm 348(1):68–80. https://doi.org/10.1002/ardp.201400271
de Rodríguez DJ, Gaytán-Sánchez NA, Rodríguez-García R, Hernández-Castillo FD, Díaz-Jiménez L et al (2019) Antifungal activity of Juglans spp. and Carya sp. ethanol extracts against Fusarium oxysporum on tomato under greenhouse conditions. Ind Crops Prod 138:111442. https://doi.org/10.1016/j.indcrop.2019.06.005
Kibret M, Guerrero-Garzón JF, Urban E, Zehl M, Wronski VK et al (2018) Streptomyces spp. from Ethiopia producing antimicrobial compounds: characterization via bioassays, genome analyses, and mass spectrometry. Front Microbiol 9:1270. https://doi.org/10.3389/fmicb.2018.01270
Polak A, Jäckel A, Noack A, Kappe R (2004) Agar sublimation test for the in vitro determination of the antifungal activity of morpholine derivatives. Mycoses 47:184–192. https://doi.org/10.1111/j.1439-0507.2004.00975.x
Titi A, Messali M, Alqurashy BA, Touzani R, Shiga T, Oshio H, Fettouhi M, Rajabi M, Almalki FA, Hadda TB (2020) Synthesis, characterization, X-ray crystal study and bioactivities of pyrazole derivatives: identification of antitumor, antifungal and antibacterial pharmacophore sites. J Mol Struct 1205:127625. https://doi.org/10.1016/j.molstruc.2019.127625
Zaki RM, Abdul-Malik MA, Saber SH, Radwan SM, El-Dean AMK (2020) A convenient synthesis, reactions and biological evaluation of novel pyrazolo[3,4-b]selenolo[3,2-e]pyrazine heterocycles as potential anticancer and antimicrobial agents. Med Chem Res 29(12):2130–2145. https://doi.org/10.1007/s00044-020-02635-z
Baldaniya BB, Patel PK (2009) Synthesis, antibacterial and antifungal activities of s-triazine derivatives. J Chem 6(3):673–680. https://doi.org/10.1155/2009/196309
Bhat M, Belagali SL (2020) Structural activity relationship and importance of benzothiazole derivatives in medicinal chemistry: a comprehensive review. Mini-Rev Org Chem 17(3):323–350. https://doi.org/10.2174/1570193X16666190204111502
Kim YJ, Kim JH, Rho JY (2019) Antifungal activities of Streptomyces blastmyceticus strain 12–6 against plant pathogenic fungi. Mycobiology 47(3):329–334. https://doi.org/10.1080/12298093.2019.1635425
Suryavanshi HR (2017) Synthesis and biological activities of piperazine derivatives as antimicrobial and antifungal agents. Org Commun 10(3):228. https://doi.org/10.25135/acg.oc.23.17.05.026
Hussain S, Ali S, Shahzadi S, Sharma SK, Qanungo K, Shahid M. Synthesis, characterization, semiempirical and biological activities of organotin (IV) carboxylates with 4-piperidinecarboxylic acid (2014) Bioinorg Chem Appl 959203. https://doi.org/10.1155/2014/959203
Li CS, Li XM, Gao SS, Lu YH, Wang BG (2013) Cytotoxic anthranilic acid derivatives from deep sea sediment-derived fungus Penicillium paneum SD-44. Mar Drug 11(8):3068–3076. https://doi.org/10.3390/md11083068
Amirkhanov NV, Tikunova NV, Pyshnyi DV (2018) Synthetic antimicrobial peptides: I. antimicrobial activity of amphiphilic and nonamphiphilic cationic peptides Rus. J Bioorganic Chem 44(5):492–503. https://doi.org/10.1134/S1068162018050035
Kondoh O, Inagaki Y, Fukuda H, Mizuguchi E, Ohya Y, Arisawa M, Shimma N, Aoki Y, Sakaitani M, Watanabe T (2005) Piperazine propanol derivative as a novel antifungal targeting 1, 3-β-D-glucan synthase. Biol Pharm Bull 28(11):2138–2141. https://doi.org/10.1248/bpb.28.2138
Carvalho A, Chu J, Meinguet C, Kiss R, Vandenbussche G, Masereel B, Wouters J, Kornienko A, Pelletier J, Mathieu V (2017) A harmine-derived beta-carboline displays anti-cancer effects in vitro by targeting protein synthesis. Eur J Pharmaco 805:25–35. https://doi.org/10.1016/j.ejphar.2017.03.034
Khabnadideh S, Rezaei Z, Pakshir K, Zomorodian K, Ghafari N (2012) Synthesis and antifungal activity of benzimidazole, benzotriazole and aminothiazole derivatives. Res Pharm Sci 7(2):65
Chohan ZH (2008) Metal-based sulfonamides: their preparation, characterization and in-vitro antibacterial, antifungal & cytotoxic properties. X-ray structure of 4-[(2-hydroxybenzylidene) amino] benzenesulfonamide (2008). J Enzyme Inhib Med Chem 23(1):120–30
Acknowledgements
Authors are grateful to Director, ICAR-NBAIM, India, for financial and infrastructural support.
Funding
This work is a part of Indian Soil Microbiome project being implemented at ICAR-NBAIM, India.
Author information
Authors and Affiliations
Contributions
HC conceptualized the study. MS, BNS, SKG, SV, PC, and SD carried out experiments and generated the data. MS, BNS, and HC prepared the manuscript draft. HC, SV, and KM revised the draft. AKS and HC edited and finalized the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The manuscript does not contain experiments involving animal or human studies.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
42770_2021_625_MOESM1_ESM.jpg
Supplementary file1 (JPG 554 KB) Supplementary Fig. 1. Antifungal activity of extracts of S. amritsarensis V31 in different solvents (ME, HE, DE, EE and CE) during well diffusion assay against fungal pathogens Alternaria alternata and Fusarium oxysporum. Here, ME=Methanolic extract, HE=Hexane extract, DE=DMSO (dimethylsulfoxide) extract, EE=Ethyl acetate extract and CE=Crude and different solvent without extract used as a control was denoted by M=Methanol, H=Hexane, D=DMSO, E=Ethyl acetate and C=Nutrient broth.
42770_2021_625_MOESM2_ESM.jpg
Supplementary file2 (JPG 841 KB) Supplementary Fig. 2. The figure represents the response of metabolite extracted from S. amritsarensis V31 on development of sheath blight disease caused by R. solani isolate PU RS1 in rice plants. R. solani isolate PU RS1 grown on PDA plate (a), rice plants grown at field (b), inoculation of sclerotia of R. solani isolate PU RS1 between leaves bases (c), moistening infected area with sterile distilled water (d) and covering of inoculated area with aseptic wet cotton (e), symptoms of sheath blight disease of rice caused by R. solani isolate PU RS1 was appeared at the infection site (in white circle) (f); and no symptom was appeared in metabolite extract sprayed before sclerotia inoculation on the rice leaves (g).
Rights and permissions
About this article
Cite this article
Shahid, M., Singh, B.N., Verma, S. et al. Bioactive antifungal metabolites produced by Streptomyces amritsarensis V31 help to control diverse phytopathogenic fungi. Braz J Microbiol 52, 1687–1699 (2021). https://doi.org/10.1007/s42770-021-00625-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00625-w