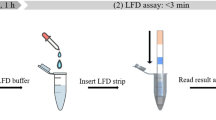
Abstract
Tuberculosis (TB) is the deadliest infectious caused by Mycobacterium tuberculosis complex (MTBC). Because most TB cases occur within low-income populations, developing a specific, sensitive, cost-saving, and rapid point-of-care test for the early diagnosis of TB is important for achieving the WHO’s End Tuberculosis Strategy. In the current study, a novel nucleic acid detection strategy that includes multiplex loop-mediated isothermal amplification combined with a nanoparticle-based lateral flow biosensor (mLAMP-LFB) was used to detect MTBC. The two sets of LAMP primers specific to the IS6110 and gyrB genes of MTBC were successfully designed and validated for the detection of MTBC. The preferred reaction conditions for this assay were confirmed to be 65 °C for 40 min, and the amplification products could be visually identified through LFB within 2 min. The full assay process, including genomic DNA template extraction, LAMP reaction, and product detection, could be completed in 80 min. The limit detection of the assay was 100 fg of DNA in pure culture. The specificity of the assay was 100%, and it had no cross-reactions to other strains. Thus, the m-LAMP-LFB technology established in the present study was an objective, rapid, simple, and sensitive assay for MTBC identification, which could be applied in a clinical setting, especially in resource-constrained regions of the world.







Similar content being viewed by others
Data availability
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.
Abbreviations
- MTBC:
-
M. tuberculosis Complex
- WHO:
-
World Health Organization
- ATCC:
-
American Type Culture Collection
- LAMP:
-
Loop-mediated isothermal amplification
- LFB:
-
Lateral flow biosensor
- MG:
-
Malachite green
- PCR:
-
Polymerase chain reaction
- LoD:
-
Limit of detection
- GZCDC:
-
Guizhou Provincial Center for Disease Control and Prevention
- FAM:
-
Carboxyfluorescein
- Dig:
-
Digoxigenin
- mer:
-
Monomeric unit
- nt:
-
Nucleotide
- CL:
-
Control line
- TL1:
-
Test line 1
- TL2:
-
Test line 2
- NC:
-
Negative control
- BC:
-
Blank control
- DW:
-
Distilled water
References
Fieweger RA, Wilburn KM, VanderVen BC (2019) Comparing the metabolic capabilities of bacteria in the Mycobacterium tuberculosis complex. Microorganisms 7(6):177
Broset E, Martín C, Gonzalo-Asensio J (2015) Evolutionary landscape of the Mycobacterium tuberculosis complex from the viewpoint of PhoPR implications for virulence regulation and application to vaccine development. mBio 6(5):1289
World Health Organization (2020) Global tuberculosis report 2019. Online at https://www.who.int/tb/publications/global_report/en/
de Martino M, Lodi L, Galli L et al (2019) Immune response to Mycobacterium tuberculosis: a narrative review. Front Pediatr 7:350
Tientcheu LD, Koch A, Ndengane M et al (2017) Immunological consequences of strain variation within the Mycobacterium tuberculosis complex. Eur J Immunol 47(3):432–445
Wilson ML (2011) Recent advances in the laboratory detection of Mycobacterium tuberculosis complex and drug resistance. Clin Infect Dis 52(11):1350–1355
Iwamoto T, Sonobe T, Hayashi K (2003) Loop-mediated isothermal amplification for direct detection of Mycobacterium tuberculosis complex, M. avium, and M. intracellulare in sputum samples. J Clin Microbiol 41(6):2616–22
Mechal Y, Benaissa E, El Mrimar N et al (2019) Evaluation of GeneXpert MTB/RIF system performances in the diagnosis of extrapulmonary tuberculosis. BMC Infect Dis 19(1):1069
Metcalf T, Soria J, Montano SM et al (2018) Evaluation of the GeneXpert MTB/RIF in patients with presumptive tuberculous meningitis. PLoS ONE 13(6):e0198695
Beavis KG, Lichty MB, Jungkind DL et al (1995) Evaluation of Amplicor PCR for direct detection of Mycobacterium tuberculosis from sputum specimens. J Clin Microbiol 33(10):2582–2586
Simner PJ, Buckwalter SP, Uhl JR et al (2013) Identification of Mycobacterium species and Mycobacterium tuberculosis complex resistance determinants by use of PCR-electrospray ionization mass spectrometry. J Clin Microbiol 51(11):3492–3498
Chen X, Ma K, Yi X et al (2020) The rapid and visual detection of methicillin-susceptible and methicillin-resistant Staphylococcus aureus using multiplex loop-mediated isothermal amplification linked to a nanoparticle-based lateral flow biosensor. Antimicrob Resist Infect Control 9(1):111
Chen X, Ma K, Yi X et al (2019) A novel detection of Enterococcus faecalis using multiple cross displacement amplification linked with gold nanoparticle lateral flow biosensor. Infect Drug Resist 12:3771–3781
Li S, Liu Y, Wang Y et al (2019) Lateral flow biosensor combined with loop-mediated isothermal amplification for simple, rapid, sensitive, and reliable detection of Brucella spp. Infect Drug Resist 12:2343–2353
Aryan E, Makvandi M, Farajzadeh A et al (2010) A novel and more sensitive loop-mediated isothermal amplification assay targeting IS6110 for detection of Mycobacterium tuberculosis complex. Microbiol Res 165(3):211–220
Raj A, Singh N, Gupta KB et al (2016) Comparative evaluation of several gene targets for designing a multiplex-PCR for an early diagnosis of extrapulmonary tuberculosis. Yonsei Med J 57(1):88–96
Liu D, He W, Jiang M et al (2019) Development of a loop-mediated isothermal amplification coupled lateral flow dipstick targeting erm(41) for detection of Mycobacterium abscessus and Mycobacterium massiliense. AMB Express 9(1):11
Wang Y, Wang Y, Quan S et al (2019) Establishment and application of a multiple cross displacement amplification coupled with nanoparticle-based lateral flow biosensor assay for detection of Mycoplasma pneumoniae. Front Cell Infect Microbiol 9:325
Cheng X, Yang J, Wang M et al (2019) Visual and rapid detection of Acinetobacter baumannii by a multiple cross displacement amplification combined with nanoparticles-based biosensor assay. AMB Express 9(1):30
Li S, Liu C, Liu Y et al (2019) Development of a multiple cross displacement amplification combined with nanoparticles-based biosensor assay to detect Neisseria meningitidis. Infect Drug Resist 12:2077–2087
Sia JK, Rengarajan J (2019) Immunology of Mycobacterium tuberculosis Infections. Microbiol Spectr 7(4):10
Guessan N, K, Horo K, Coulibaly I, et al (2016) Rapid detection of Mycobacterium tuberculosis complex in sputum samples using PURE TB-LAMP assay. Int J Mycobacteriol Suppl 1:S164–S165
Spositto FL, Campanerut PA, Ghiraldi LD et al (2014) Multiplex-PCR for differentiation of Mycobacterium bovis from Mycobacterium tuberculosis complex. Braz J Microbiol 45(3):841–843
Xu HB, Jiang RH, Sha W et al (2010) PCR-single-strand conformational polymorphism method for rapid detection of rifampin-resistant Mycobacterium tuberculosis: systematic review and meta-analysis. J Clin Microbiol 48(10):3635–3640
Pang Y, Shang Y, Lu J et al (2017) GeneXpert MTB/RIF assay in the diagnosis of urinary tuberculosis from urine specimens. Sci Rep 7(1):6181
Reddy R, Alvarez-Uria G (2017) Molecular epidemiology of rifampicin resistance in Mycobacterium tuberculosis using the GeneXpert MTB/RIF assay from a rural setting in India. J Pathog 2017:6738095
Wang Y, Li H, Wang Y et al (2017) Loop-mediated isothermal amplification label-based gold nanoparticles lateral flow biosensor for detection of Enterococcus faecalis and Staphylococcus aureus. Front Microbiol 8:192
Angkasekwinai N, Atkins EH, Johnson RN et al (2014) Rapid and sensitive detection of Bartonella bacilliformis in experimentally infected sand flies by loop-mediated isothermal amplification (LAMP) of the Pap31 gene. PLoS Negl Trop Dis 8(12):e3342
Niemann S, Harmsen D, Rüsch-Gerdes S et al (2000) Differentiation of clinical Mycobacterium tuberculosis complex isolates by gyrB DNA sequence polymorphism analysis. J Clin Microbiol 38(9):3231–3234
Yin XM, Wu LJ, Zheng L et al (2016) Quantification of colony-forming units for M. tuberculosis complex using gyrB-based real-time PCR assay. Int J Tuberc Lung Dis 20(7):967–72
Huang W, Zhang H, Xu J et al (2017) Loop-mediated isothermal amplification method for the rapid detection of Ralstonia solanacearum phylotype I mulberry strains in China. Front Plant Sci 8:76
Law JW, Ab Mutalib NS, Chan KG et al (2015) Rapid methods for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front Microbiol 5:770
Acknowledgements
We acknowledge the medical staff at the Guizhou Provincial Center for Disease Control and Prevention for their cooperation in this study.
Funding
This study was funded by grants from the Science and Technology Department of Guizhou Province (No. [2019]1186, [2019]2822), [2018]-5606, and [2018]-5767).
Author information
Authors and Affiliations
Contributions
XC and SL designed and conceived the experiments. XC, JH, and SL designed and analyzed the LAMP primers. XC, JH, ZX, XY, and YC performed the experiments. YC, WZ, WC, and HC collected and analyzed the data. XC and SL wrote and revised the manuscript. XC and SL revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Human Ethics Committee of the Guizhou Provincial Center for Disease Control and Prevention and complied with the Declaration of Helsinki. All data/isolates were analyzed anonymously. The patients with tuberculosis included in the present research were given a subject information sheet, and they all provided written informed consent before participating in the study.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Fernando R. Pavan
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, X., Huang, J., Xiao, Z. et al. Highly specific and sensitive detection of the Mycobacterium tuberculosis complex using multiplex loop-mediated isothermal amplification combined with a nanoparticle-based lateral flow biosensor. Braz J Microbiol 52, 1315–1325 (2021). https://doi.org/10.1007/s42770-021-00520-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00520-4