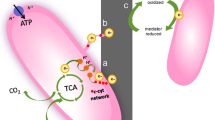
Abstract
Microbial life is predominantly observed as biofilms, which are a sessile aggregation of microbial cells formed in response to stress conditions. The microtiter dish biofilm formation assay is one of the most important methods of studying biofilm formation. In this study, the assay has been improvised to allow easy detection of biofilm formation on different substrata. The method has then been used to study growth conditions that affect biofilm formation, viz., the effect of pH, temperature, shaking conditions, and the carbon source provided. Glass, cellulose acetate, and carbon cloth materials were used as substrata to study biofilm development under the above conditions. The method was then extended to determine biofilm formation on the anodes of a microbial fuel cell in order to study the effect of biofilm formation on power production. A high correlation was observed between biofilm formation and power density (r = 0.951). When the electrode containing a biofilm was replaced with another electrode without biofilm, the average power density dropped from 59.55 to 5.76 mW/m2. This method offers an easy way to study the suitability of different materials to support biofilm formation. Growth conditions determining biofilm formation can be studied using this method. This method also offers a non-invasive way to determine biofilm formation on anodes of microbial fuel cells and preserves the anode for further studies.





Similar content being viewed by others
References
Merritt JH, Kadouri DE, O’Toole GA (2011) Growing and analyzing static biofilms. Curr Protoc Microbiol 22(SUPPL 22):1–18. https://doi.org/10.1002/9780471729259.mc01b01s22
Flemming H-C, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S (2016) Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14(9):563–575. https://doi.org/10.1038/nrmicro.2016.94
Jefferson KK (2004) What drives bacteria to produce a biofilm? FEMS Microbiol Lett 236(2):163–173. https://doi.org/10.1016/j.femsle.2004.06.005
Kumar R, Singh L, Wahid ZA, Din MFM (2015) Exoelectrogens in microbial fuel cells toward bioelectricity generation: a review. Int J Energy Res 39(8):1048–1067. https://doi.org/10.1002/er.3305
Sutherland IW (2001) The biofilm matrix - an immobilized but dynamic microbial environment. Trends Microbiol 9(5):222–227. https://doi.org/10.1016/S0966-842X(01)02012-1
Wilkins M, Hall-Stoodley L, Allan RN, Faust SN (2014) New approaches to the treatment of biofilm-related infections. J Infect 69(S1):S47–S52. https://doi.org/10.1016/j.jinf.2014.07.014
Percival SL, Suleman L, Donelli G (2015) Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. J Med Microbiol 64(4):323–334. https://doi.org/10.1099/jmm.0.000032
Aiyer KS, Vijayakumar BS, Vishwanathan AS (2018) The enigma of biofilms. Curr Sci 115(2):204–205
Falkowski PG, Fenchel T, Delong EF (2008) The microbial engines that drive Earth’s biogeochemical cycles. Science 320(5879):1034–1039. https://doi.org/10.1126/science.1153213
Godia F, Sola C (1995) Fluidized-bed bioreactors. Biotechnol Prog 11(5):479–497. https://doi.org/10.1021/bp00035a001
Logan BE, Hamelers B, Rozendal R, Schröder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K (2006) Microbial fuel cells: methodology and technology †. Environ Sci Technol 40(17):5181–5192. https://doi.org/10.1021/es0605016
Gude VG (2016) Wastewater treatment in microbial fuel cells – an overview. J Clean Prod 122:287–307. https://doi.org/10.1016/j.jclepro.2016.02.022
Azeredo J, Azevedo NF, Briandet R, Cerca N, Coenye T, Costa AR, Desvaux M, di Bonaventura G, Hébraud M, Jaglic Z, Kačániová M, Knøchel S, Lourenço A, Mergulhão F, Meyer RL, Nychas G, Simões M, Tresse O, Sternberg C (2017) Critical review on biofilm methods. Crit Rev Microbiol 43(3):313–351. https://doi.org/10.1080/1040841X.2016.1208146
Pettit RK, Weber CA, Kean MJ, Hoffmann H, Pettit GR, Tan R, Franks KS, Horton ML (2005) Microplate Alamar blue assay for Staphylococcus epidermidis biofilm susceptibility testing. Antimicrob Agents Chemother 49(7):2612–2617. https://doi.org/10.1128/AAC.49.7.2612-2617.2005
Donovan C, Dewan A, Heo D, Beyenal H (2008) Batteryless, wireless sensor powered by a sediment microbial fuel cell. Environ Sci Technol 42(22):8591–8596. https://doi.org/10.1021/es801763g
Feoktistova M, Geserick P, Leverkus M (2016) Crystal violet assay for determining viability of cultured cells. Cold Spring Harb Protoc 2016(4):343–346. https://doi.org/10.1101/pdb.prot087379
Beecroft NJ, Zhao F, Varcoe JR, Slade RCT, Thumser AE, Avignone-Rossa C (2012) Dynamic changes in the microbial community composition in microbial fuel cells fed with sucrose. Appl Microbiol Biotechnol 93(1):423–437. https://doi.org/10.1007/s00253-011-3590-y
O’Toole GA (2011) Microtiter dish biofilm formation assay. J Vis Exp (47):3–5. https://doi.org/10.3791/2437
Bower CK, Mcguire J, Daeschel MA (1996) The adhesion and detachment of bacteria and spores on food-contact surfaces. Trends Food Sci Technol 7(5):152–157. https://doi.org/10.1016/0924-224481255-6
Lobelle D, Cunliffe M (2011) Early microbial biofilm formation on marine plastic debris. Mar Pollut Bull 62(1):197–200. https://doi.org/10.1016/j.marpolbul.2010.10.013
Burton E, Yakandawala N, LoVetri K, Madhyastha MS (2007) A microplate spectrofluorometric assay for bacterial biofilms. J Ind Microbiol Biotechnol 34(1):1–4. https://doi.org/10.1007/s10295-006-0086-3
Billings N, Birjiniuk A, Samad TS, Doyle PS, Ribbeck K (2015) Material properties of biofilms—a review of methods for understanding permeability and mechanics. Rep Prog Phys 78(3):036601. https://doi.org/10.1088/0034-4885/78/3/036601
Stoodley P, DeBeer D, Lappin - Scott H (1997) Influence of electric fields and pH on biofilm structure as related to the bioelectric effect. Antimicrob Agents Chemother 41(9):1876–1879
Harvey J, Keenan KP, Gilmour A (2007) Assessing biofilm formation by listeria monocytogenes strains. Food Microbiol 24(4):380–392. https://doi.org/10.1016/j.fm.2006.06.006
Moreira JMR, Gomes LC, Araújo JDP, Miranda JM, Simões M, Melo LF, Mergulhão FJ (2013) The effect of glucose concentration and shaking conditions on Escherichia coli biofilm formation in microtiter plates. Chem Eng Sci 94:192–199. https://doi.org/10.1016/j.ces.2013.02.045
Gristina AG, Costerton JW (1985) Bacterial adherence to biomaterials and tissue. The significance of its role in clinical sepsis. J Bone Joint Surg 67(2):264–273. https://doi.org/10.2106/00004623-198567020-00014
Villaverde S, García-Encina PA, Fdz-Polanco F (1997) Influence of pH over nitrifying biofilm activity in submerged biofilters. Water Res 31(5):1180–1186. https://doi.org/10.1016/S0043-1354(96)00376-4
Römling U, Kjelleberg S, Normark S, Nyman L, Uhlin BE, Åkerlund B (2014) Microbial biofilm formation: a need to act. J Intern Med 276(2):98–110. https://doi.org/10.1111/joim.12242
Peyton BM (1996) Effects of shear stress and substrate loading rate on Pseudomonas aeruginosa biofilm thickness and density. Water Res 30(1):29–36. https://doi.org/10.1016/0043-1354(95)00110-7
Stoodley P, Dodds I, Boyle JD, Lappin-Scott HM (1998) Influence of hydrodynamics and nutrients on biofilm structure. J Appl Microbiol 85(S1):19S–28S. https://doi.org/10.1111/j.1365-2672.1998.tb05279.x
Stepanović S, Vuković D, Dakić I, Savić B, Švabić-Vlahović M (2000) A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods 40(2):175–179. https://doi.org/10.1016/S0167-7012(00)00122-6
Mohamed JA, Huang DB (2007) Biofilm formation by enterococci. J Med Microbiol 56(12):1581–1588. https://doi.org/10.1099/jmm.0.47331-0
van der Kooij D, Veenendaal HR, Baars-Lorist C, van der Klift DW, Drost YC (1995) Biofilm formation on surfaces of glass and Teflon exposed to treated water. Water Res 29(7):1655–1662. https://doi.org/10.1016/0043-1354(94)00333-3
Rochex A, Lebeault J-M (2007) Effects of nutrients on biofilm formation and detachment of a Pseudomonas putida strain isolated from a paper machine. Water Res 41(13):2885–2892. https://doi.org/10.1016/j.watres.2007.03.041
Duetz WA, Witholt B (2004) Oxygen transfer by orbital shaking of square vessels and deepwell microtiter plates of various dimensions. Biochem Eng J 17(3):181–185. https://doi.org/10.1016/S1369-703X(03)00177-3
Kumamoto CA (2002) Candida biofilms. Curr Opin Microbiol 5(6):608–611. https://doi.org/10.1016/S1369-5274(02)00371-5
Stepanović S, Ćirković I, Mijač V, Švabić-Vlahović M (2003) Influence of the incubation temperature, atmosphere and dynamic conditions on biofilm formation by Salmonella spp. Food Microbiol 20(3):339–343. https://doi.org/10.1016/S0740-0020(02)00123-5
Kumar R, Singh L, Zularisam AW, Hai FI (2018) Microbial fuel cell is emerging as a versatile technology: a review on its possible applications, challenges and strategies to improve the performances. Int J Energy Res 42(2):369–394. https://doi.org/10.1002/er.3780
Xiao Y, Zhao F (2017) Electrochemical roles of extracellular polymeric substances in biofilms. Curr Opin Electrochem 4(1):206–211. https://doi.org/10.1016/j.coelec.2017.09.016
Patil SA, Hägerhäll C, Gorton L (2012) Electron transfer mechanisms between microorganisms and electrodes in bioelectrochemical systems. In: Advances in chemical bioanalysis, vol 1. Springer International Publishing, Cham, pp 71–129. https://doi.org/10.1007/11663_2013_2
Schröder U (2007) Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency. Phys Chem Chem Phys 9(21):2619–2629. https://doi.org/10.1039/b703627m
Logan BE (2009) Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol 7(5):375–381. https://doi.org/10.1038/nrmicro2113
Saratale GD, Saratale RG, Shahid MK, Zhen G, Kumar G, Shin H-S, Choi YG, Kim S-H (2017) A comprehensive overview on electro-active biofilms, role of exo-electrogens and their microbial niches in microbial fuel cells (MFCs). Chemosphere 178:534–547. https://doi.org/10.1016/j.chemosphere.2017.03.066
Reguera G, Nevin KP, Nicoll JS, Covalla SF, Woodard TL, Lovley DR (2006) Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl Environ Microbiol 72(11):7345–7348. https://doi.org/10.1128/AEM.01444-06
Acknowledgments
The authors dedicate this work to Bhagawan Sri Sathya Sai Baba, the founder chancellor of Sri Sathya Sai Institute of Higher Learning.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
N/A
Additional information
Responsible Editor: Vania M.M. Melo.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aiyer, K.S., Vijayakumar, B.S. An improvised microtiter dish biofilm assay for non-invasive biofilm detection on microbial fuel cell anodes and studying biofilm growth conditions. Braz J Microbiol 50, 769–775 (2019). https://doi.org/10.1007/s42770-019-00091-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-019-00091-5