Abstract
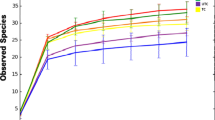
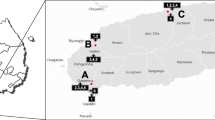
Elucidation of the distinctive microbial taxonomic profiles of tropical fruit peels is the indispensable component of investigations aimed at the detection of microorganisms responsible for the post-harvest loss. The objective of the present work was to dissect the bacterial and fungal community of five tropical fruit peels (banana, guava, mango, papaya, and passion fruit) in wild (non-cultivated) and conventionally produced samples from Brazil. To that end, 16S rRNA–encoding gene and ITS rDNA amplicon analysis of the five tropical fruit peels were performed to discriminate the bacterial and fungal communities, respectively. The result showed that bacterial communities of the five types of fruit peels were by far more diversified than that of fungal communities, independent of the type of production system involved. Among the investigated fruits, non-cultivated papaya peels hosted the most diversified bacterial community while the least bacterial community diversity was found in the conventionally produced papaya fruit peels. The gene amplicon analysis clearly discriminated the bacterial community into their respective classes, while fungal communities were better classified in their phyla, yet with clearer component discrimination of fungal community based on the type of cultivation system practiced. Conventionally produced banana and non-cultivated passion fruit peels were characteristically dominated by fungal and bacterial groups, respectively. Overall, in conventionally produced fruit peels, bacterial community was mainly composed of Proteobacteria, Actinobacteria, and Bacilli. The result provided a broad microbial diversity profile that could be used as an important input for seeking alternative fruit spoilage control and post-harvest treatments.






Similar content being viewed by others
Change history
30 November 2019
The original version of this article unfortunately contained two mistakes in the “Materials and methods” section, subsection “DNA extraction and PCR” of the article. The correct information is given below.
30 November 2019
The original version of this article unfortunately contained two mistakes in the ���Materials and methods��� section, subsection ���DNA extraction and PCR��� of the article. The correct information is given below.
References
Scherrer J (2018) The Most popular fruit in the world. Bandeiras News
Onyeani CA, Osunlaj SO, Owuru OO, Sosanya OS (2012) Mango fruit anthracnose and the effects on mango yield and market values in southwestern Nigeria. Asian J Agric Res 6(4):171–179. https://doi.org/10.3923/ajar.2012.171.179.
Udayanga D, Manamgoda DS, Liu X, Chukeatirote E, Hyde KD (2013) What are the common anthracnose pathogens of tropical fruits? Fungal Divers 61(1):165–179. https://doi.org/10.1007/s13225-013-0257-2
Wang Z, Cui Y, Vainstein A, Chen S, Ma H (2017) Regulation of fig (Ficus carica L.) fruit color: metabolomic and transcriptomic analyses of the flavonoid biosynthetic pathway. Front Plant Sci 8:1990. https://doi.org/10.3389/fpls.2017.01990
Abdelfattah A, Wisniewski M, Droby S, Schena L (2016) Spatial and compositional variation in the fungal communities of organic and conventionally grown apple fruit at the consumer point-of-purchase. Hortic Res 3(1):16047. https://doi.org/10.1038/hortres.2016.47
Mandal D, Sequenc NG, De Mandal S, Panda AK, Bisht SS, Kumar NS (2015) Microbial ecology in the era of next generation sequencing. Next Gener Seq Appl:1–6. https://doi.org/10.4172/jngsa.S1-001
Kõljalg U, Nilsson RH, Abarenkov K, Tedersoo L, Taylor AFS, Bahram M, Bates ST, Bruns TD, Bengtsson-Palme J, Callaghan TM, Douglas B, Drenkhan T, Eberhardt U, Dueñas M, Grebenc T, Griffith GW, Hartmann M, Kirk PM, Kohout P, Larsson E, Lindahl BD, Lücking R, Martín MP, Matheny PB, Nguyen NH, Niskanen T, Oja J, Peay KG, Peintner U, Peterson M, Põldmaa K, Saag L, Saar I, Schüßler A, Scott JA, Senés C, Smith ME, Suija A, Taylor DL, Telleria MT, Weiss M, Larsson KH (2013) Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol 22(21):5271–5277. https://doi.org/10.1111/mec.12481
Diniz LEC, Sakiyama NS, Lashermes P, Caixeta ET, Oliveira ACB, Zambolim EM, Loureiro ME, Pereira AA, Zambolim L (2005) Analysis of AFLP markers associated to the Mex-1 resistance locus in Icatu progenies. Crop Breed Appl Biotechnol 5(4):387–393. https://doi.org/10.12702/1984-7033.v05n04a03
Rastogi G, Tech JJ, Coaker GL, Leveau JHJ (2010) A PCR-based toolbox for the culture-independent quantification of total bacterial abundances in plant environments. J Microbiol Methods 83(2):127–132. https://doi.org/10.1016/j.mimet.2010.08.006
Buée M, Reich M, Murat C, Morin E, Nilsson RH, Uroz S, Martin F (2009) 454 pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. New Phytol 184(2):449–456. https://doi.org/10.1111/j.1469-8137.2009.03003.x
Angiuoli SV, Matalka M, Gussman A, Galens K, Vangala M, Riley DR, Arze C, White JR, White O, Fricke WF (2011) CloVR: a virtual machine for automated and portable sequence analysis from the desktop using cloud computing. BMC Bioinformatics 12(1):356. https://doi.org/10.1186/1471-2105-12-356
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. https://doi.org/10.1038/nmeth.f.303
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 26:2460–2461. https://doi.org/10.1093/bioinformatics/btq461
Oudah M, Henschel A (2018) Taxonomy-aware feature engineering for microbiome classification. BMC Bioinformatics 19(1):227. https://doi.org/10.1186/s12859-018-2205-3
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. https://doi.org/10.1128/AEM.01541-09
Saminathan T, García M, Ghimire B, Lopez C, Bodunrin A, Nimmakayala P, Abburi VL, Levi A, Balagurusamy N, Reddy UK (2018) Metagenomic and metatranscriptomic analyses of diverse watermelon cultivars reveal the role of fruit associated microbiome in carbohydrate metabolism and ripening of mature fruits. Front Plant Sci 9:4. https://doi.org/10.3389/fpls.2018.00004
Narayanasamy P (2011) Detection of fungal pathogens in plants. In: Phangal Pathogens. Springer. doi:https://doi.org/10.1007/978-90-481-9735-4_2.
Reiter B, Sessitsch A (2006) Bacterial endophytes of the wildflower Crocus albiflorus analyzed by characterization of isolates and by a cultivation-independent approach. Can J Microbiol 52(2):140–149. https://doi.org/10.1139/w05-109
Leff JW, Noah F (2013) Bacterial communities associated with the surfaces of fresh fruits and vegetables. PLoS One 8(3):e59310. https://doi.org/10.3389/fpls.2018.00004
Sengupta S, Ganguli S, Singh PK (2017) Metagenome analysis of the root endophytic microbial community of Indian rice ( O. sativa L.). Genomics Data 12:41–43. https://doi.org/10.1016/j.gdata.2017.02.010
Barth M, Hankinson TR, Zhuang H, Breidt F (2009) Microbiological spoilage of fruits and vegetables. In: Compendium of the microbiological spoilage of foods and beverages. Springer New York, New York, NY, pp 135–183. https://doi.org/10.1007/978-1-4419-0826-1_6
Ezra D, Kirshner B, Hershcovich M, Shtienberg D, Kosto I (2015) Heart rot of pomegranate: disease etiology and the events leading to development of symptoms. Plant Dis 99(4):496–501. https://doi.org/10.1094/PDIS-07-14-0707-RE
Schmid F, Moser G, Müller H, Berg G (2011) Functional and structural microbial diversity in organic and conventional viticulture: organic farming benefits natural biocontrol agents. Appl Environ Microbiol 77(6):2188–2191. https://doi.org/10.1128/AEM.02187-10
Suriya J, Bharathiraja S, Manivasagan P, Kim S-K (2016) Enzymes rrom rare actinobacterial strains. In 67–98. doi:https://doi.org/10.1016/bs.afnr.2016.08.002.
Joana Gil-Chávez G, Villa JA, Fernando Ayala-Zavala J, Basilio Heredia J, Sepulveda D, Yahia EM, González-Aguilar GA (2013) Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: an overview. Compr Rev Food Sci Food Saf 12(1):5–23. https://doi.org/10.1111/1541-4337.12005
Sagar NA, Pareek S, Sharma S, Yahia EM, Lobo MG (2018) Fruit and vegetable waste: bioactive compounds, their extraction, and possible utilization. Compr Rev Food Sci Food Saf 17:512–531. https://doi.org/10.1111/1541-4337.12330
Muthusamy S, Selvan ST, Arunachalam P, Grasian I (2017) Bioconversion and bioethanol production from agro-residues through fermentation process using mangrove-associated actinobacterium Streptomyces olivaceus (MSU3). Biofuels.:1–13. https://doi.org/10.1080/17597269.2017.1309853
Shen Y, Nie J, Li Z, Li H, Wu Y, Dong Y, Zhang J (2018) Differentiated surface fungal communities at point of harvest on apple fruits from rural and peri-urban orchards. Sci Rep 8(1):2165. https://doi.org/10.1038/s41598-017-17436-5
Pinto CC, Pinho D, Sousa S, Pinheiro M, Egas C, C. Gomes A (2014) Unravelling the diversity of grapevine microbiome. Driks A, ed. PLoS One;9(1):e85622. doi:https://doi.org/10.1371/journal.pone.0085622.
Singh S, Gupta R, Kumari M, Sharma S (2015) Nontarget effects of chemical pesticides and biological pesticide on rhizospheric microbial community structure and function in Vigna radiata. Environ Sci Pollut Res 22(15):11290–11300. https://doi.org/10.1007/s11356-015-4341-x
Rytioja J, Hildén K, Yuzon J, Hatakka A, de Vries RP, Mäkelä MR (2014) Plant-polysaccharide-degrading enzymes from Basidiomycetes. Microbiol Mol Biol Rev 78(4):614–649. https://doi.org/10.1128/MMBR.00035-14
Rathee S, Rathee D, Rathee D, Kumar V, Rathee P (2012) Mushrooms as therapeutic agents. Brazilian J Pharmacogn 22:459–474. https://doi.org/10.1590/S0102-695X2011005000195
Earl JP, Adappa ND, Krol J, Bhat AS, Balashov S, Ehrlich RL, Palmer JN, Workman AD, Blasetti M, Sen B, Hammond J, Cohen NA, Ehrlich GD, Mell JC (2018) Species-level bacterial community profiling of the healthy sinonasal microbiome using Pacific Biosciences sequencing of full-length 16S rRNA genes. Microbiome. 6(1):190. https://doi.org/10.1186/s40168-018-0569-2
Blaalid R, Kumar S, Nilsson RH, Abarenkov K, Kirk PM, Kauserud H (2013) ITS1 versus ITS2 as DNA metabarcodes for fungi. Mol Ecol Resour 13(2):218–224. https://doi.org/10.1111/1755-0998.12065
Acknowledgments
This project was supported by the Alexander von Humboldt-Foundation—AvH (Germany) and National Council for Scientific and Technological Development—CNPq (Brazil). Thanks to the Cell Biology Department, Universidade de Brasilia, for allowing us to use their facilities during the DNA extraction.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Luis Augusto Nero
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cruz, A.F., Barka, G.D., Blum, L.E.B. et al. Evaluation of microbial communities in peels of Brazilian tropical fruits by amplicon sequence analysis. Braz J Microbiol 50, 739–748 (2019). https://doi.org/10.1007/s42770-019-00088-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-019-00088-0