Abstract
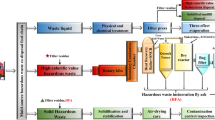
In this study, the method of fluidized incineration and water washing to recover hydrogen fluoride (HF) was proposed to dispose of high fluorine-containing organic waste. The resource utilization of the waste was investigated in a fluidized bed incinerator with a disposal capability of 10 t/d. The evolution characteristics of fluorine, operation conditions of the incineration system, absorption coefficient for HF by water washing, and HF corrosion during combustion were assessed. The results showed that HF and fluorocarbons were detected as the initial gaseous fluorides released during combustion. The release of HF could be divided into three stages, in which HF was generated from the volatilization of HF in the waste and the hydrolysis of fluorine in water-soluble salts (60–220 °C), oxidative decomposition of fluorinated organic components and residual carbon (220–800 °C), and hydrolysis of insoluble fluorinated inorganic minerals (800–1000 °C). Fluorocarbons could be destroyed through reactions with free radicals H, O, and OH or through single-molecule decomposition. Enhancing the temperature in the furnace and increasing the content of oxygen and hydrogen in the incineration materials were conducive to reducing the generation of fluorocarbons. By sampling and analyzing the bottom slag, bag filter ash and exhaust gas during the field test, the relevant pollutant discharge could meet the national emission standards. The waste heat utilization of high-temperature flue gas and the recovery of hydrofluoric acid and hydrochloric acid were realized. In the recovery of HF by water washing, the total absorption coefficients for 1# to 4# packed absorbers were 52.38 kg/(h m2), 39.96 kg/(h m2), 5.98 kg/(h m2) and 3.89 kg/(h m2), respectively. In the actual operation, alumina showed good corrosion resistance to high-temperature HF and could be used as bed materials or refractory materials. Low-temperature corrosion of HF occurred in the quenching heat exchanger, which was damaged after 6 months of continuous operation. High-temperature corrosion of HF occurred in the waste heat boiler. No significant corrosion was observed in the 24 months of operation.










Similar content being viewed by others
Change history
11 April 2023
A Correction to this paper has been published: https://doi.org/10.1007/s42768-023-00146-2
References
Orloff, K., and Falk, H. 2003. An international perspective on hazardous waste practices. International Journal of Hygiene and Environmental Health 206 (4–5): 291–302.
Zhu, H.M., Yan, J.H., Jiang, X.G., et al. 2008. Study on pyrolysis of typical medical waste materials by using TG-FTIR analysis. Journal of Hazardous Materials 153 (1): 670–676.
Feng, Y., Jiang, X., and Chen, D. 2016. The emission of fluorine gas during incineration of fluoroborate residue. Journal of Hazardous Materials 308: 91–96.
Feng, Y.H., Jiang, X.G., Chi, Y., et al. 2011. Volatilization behavior of fluorine in fluoroborate residue during pyrolysis. Environmental Science & Technology 46 (1): 307–311.
Ministry of Environmental Protection. 2005. Technical specifications for Centralized Incineration Facility. HJ/T176. Beijing, China: MEP.
Qi, Q. 2002. The Mechanism and experimental studies on occurrence characteristics, combustion transfer and pollution control technology of fluorine in coal. Ph.D. dissertation. Hangzhou, China: Zhejiang University (in Chinese).
Guo, S., Yang, J., and Liu, Z. 2006. The fate of fluorine and chlorine during thermal treatment of coals. Environmental Science & Technology 40 (24): 7886–7889.
Li, W., Lu, H.L., Chen, H.K., et al. 2005. Volatilization behavior of fluorine in coal during fluidized-bed pyrolysis and CO2-gasification. Fuel 84 (4): 353–357.
Liu, J.Z., Qi, Q.J., Sheng, J.J., et al. 2004. Experimental study on fluorine retention process and additive under high temperature during coal combustion. Proceedings of the CSEE 07: 231–234 (in Chinese).
Qi, Q., Liu, J., Cao, X. et al. 2003. Fluorine emission characteristics and kinetic mechanism during coal combustion. Journal of Fuel Chemistry and Technology 31 (5): 400–404 (in Chinese).
Qi, Q., Liu, J.Z., Zhou, J.H., et al. 2003. Fluoride emission control by blending and injecting CaO and Calcium based sorbents during coal combustion. Journal of chemical industry and Engineering 02: 226–231 (in Chinese).
García-Ten, J., Monfort, E., Tena, M.P.G., et al. 2006. Influence of calcite content on fluorine compound emissions during ceramic tile firing. Journal of Ceramic Processing & Research 7 (1): 75–82.
Brosnan, D. 1992. Technology and regulatory consequences of fluorine emissions in ceramic manufacturing. American Ceramic Society Bulletin 71 (12): 1798–1802.
González, I., Galán, E., and Miras, A. 2006. Fluorine, chlorine and sulphur emissions from the Andalusian ceramic industry (Spain)—Proposal for their reduction and estimation of threshold emission values. Applied Clay Science 32 (3–4): 153–171.
Monfort, E., García-Ten, J., Celades, I., et al. 2008. Evolution of fluorine emissions during the fast firing of ceramic tile. Applied Clay Science 38 (3–4): 250–258.
Swaine, D. 1990. Trace elements in coal. Trace elements in coal, 27–49.
Shin, M., Jang, D., and Ha, J. 2016. Experimental and numerical calculations of waste R-134a thermal decomposition for energy and fluorine material recovery. Journal of Material Cycles and Waste Management 18 (3): 399–406.
Ritter, E.R. 1994. Experimental study on Freon 113 decomposition under inert and reducing conditions. Combustion Science and Technology 101: 171–186.
Wang, F., Lu, X.W., Li, X.Y., et al. 2015. Effectiveness and mechanisms of defluorination of perfluorinated alkyl substances by calcium compounds during waste thermal treatment. Environmental Science & Technology 49 (9): 5672–5680.
Ministry of Environmental Protection. 2014. Determination of Fluorine in coal. GB/T4633. Beijing, China: MEP.
Li, C., and Suzuki, K. 2009. Kinetic analyses of biomass tar pyrolysis using the distributed activation energy model by TG/DTA technique. Journal of Thermal Analysis and Calorimetry 98 (1): 261–266.
Lv, P., Sun, X.-G., Sun, H., et al. 2013. Infrared characterization of fluorine-containing gas of 1-chloro-2,2-difluoroethylene fluoride and hexafluoro-2-butyne. Zhejiang Chemical Industry 44 (01): 38–41 (in Chinese).
Burgess, D.R., Jr., Zachariah, M.R., Tsang, W., et al. 1995. Thermochemical and chemical kinetic data for fluorinated hydrocarbons. Progress in Energy and Combustion Science 21 (6): 453–529.
Ministry of Environmental Protection. 2001. Standard for pollution control on hazardous waste incineration, GB18484. Beijing, China: MEP.
Vieweg, R. 1963. Examination of the system HF−H2O. Chemical Technology (Berlin) 12 (15): 734–740.
Yao, Y., Xia, Q., Chen, C.G., et al. 2005. Principles of chemical engineering. Tianjin, China: Tianjin University Press (in Chinese).
Lu, Z. 2000. Analysis of hydrogen fluoride absorption process. Light Metal 09: 15–19 (in Chinese).
Lamar. 1955. Absorption Operation in Chemical Industry. Beijing, China: Higher Education Press (in Chinese).
Lgamri, A., Guenbour, A., Bachir, A.B., et al. 2003. Characterisation of electrolytically deposited alumina and yttrium modified alumina coatings on steel. Surface & Coatings Technology 162 (2): 154–160.
Ritala, H., Kiihamaki, J., and Heikkila, M. 2010. Studies on aluminium corrosion during and after HF vapour treatment. Microelectronic Engineering 87 (3): 501–504.
Gao, X., Liu, J.Y., and Liu, J. 2011. High temperature fluoride corrosion resistance of aluminum-containing high chromium cast iron. Jinsu Rechuli 36 (02): 10–13 (in Chinese).
Linstrom, P.J., and Mallard, W.G. 2001. The NIST chemistry webbook: a chemical data resource on the internet. Journal of Chemical & Engineering Data 46 (5): 1059–1063.
Acknowledgements
This study was supported by the Major Science and Technology Projects of Zhejiang Province (No. 2020C03087).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Jianhua Yan is the Editor-in-Chief of Waste Disposal & Sustainable Energy. On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised to correct the Conflict of Interest section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, W., Ma, Z., Yan, J. et al. Study on the resource utilization of high fluorine-containing organic waste through fluidized incineration. Waste Dispos. Sustain. Energy 4, 117–129 (2022). https://doi.org/10.1007/s42768-022-00101-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42768-022-00101-7