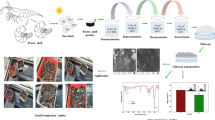
Abstract
In the present study, we extracted and characterized chitin of two storage pests, Tribolium castaneum and Sitophilus oryzae and compared their physicochemical properties with commercial shrimp chitin. For the characterization, we performed Fourier transform infrared spectroscopy (FT-IR), X-ray diffractometry (XRD), Elemental analysis (EA), reducing sugar assay, and scanning electron microscopy (SEM). The percentage of yield was 26.38% and 32.72% for T.castaneum and S.oryzae respectively. FT-IR and XRD data evaluated that the chitin is in its alpha form. The Crystalline Index (CrI 110) of chitin extracted from T. castaneum and S. oryzae were 81% and 82% and are close to that of commercial chitin of shrimp. SEM showed a porous and fibrous structure in S. oryzae whereas in T. castaneum it was planer and less porous. The degree of Acetylation (DA) values was calculated as 57.67% for T. castaneum and 123.54% for S. oryzae in accordance with the elemental content results. Reducing sugar assay results depicted that the chitin of both the insects is almost similar to commercial quality. The data obtained in current study can be used for researchers working on T. castaneum and S. oryzae.





Similar content being viewed by others
References
Andonegi M, Heras KL, Santos-Vizcaíno E, Igartua M, Hernandez RM, de la Caba K, Guerrero P (2020) Structure-properties relationship of chitosan/collagen films with potential for biomedical applications. Carbohydr Polym 237:116159. https://doi.org/10.1016/j.carbpol.2020.116159
Antunes C, Mendes R, Lima A, Barros G, Fields P, Da Costa LB, Rodrigues JC, Silva MJ, Correia AM, Carvalho MO (2016) Resistance of Rice Varieties to the stored-product insect, Sitophilus zeamais (Coleoptera: Curculionidae). J Econ Entomol 109(1):445–453. https://doi.org/10.1093/jee/tov260
Arakane Y, Muthukrishnan S, Kramer KJ, Specht CA, Tomoyasu Y, Lorenzen MD, Kanost M, Beeman RW (2005) The Tribolium chitin synthase genes TcCHS1 and TcCHS2 are specialized for synthesis of epidermal cuticle and midgut peritrophic matrix. Insect Mol Biol 14(5):453–463. https://doi.org/10.1111/j.1365-2583.2005.00576.x
Aranaz I, Mengibar M, Harris R, Panos I, Miralles B, Acosta N, Galed G, Heras A (2009) Functional characterization of chitin and chitosan. Curr Chem Biol 3:203–230
Bry RE, Davis R (1985) Tribolium confusum and Tribolium castaneum. In: Singh P, Moore RF (eds) Handbook of insect rearing, vol 1. Elsevier, Amsterdam-Oxford-New York-Tokyo, pp 291–293
Chandran R, Williams L, Hung A, Nowlin K, LaJeunesse D (2016) SEM characterization of anatomical variation in chitin organization in insect and arthropod cuticles. Micron 82:74–85. https://doi.org/10.1016/j.micron.2015.12.010
Chaussard G, Domard A (2004) New Aspects of the extraction of chitin from Squid Pens. Biomacromolecules 5(2):559–564. https://doi.org/10.1021/bm034401t
Derbalah AS, Hamza AM, Gazzy AA (2012) Efficacy and safety of some plant extracts as alternatives for Sitophilus oryzae control in rice grains. J Entomo l9(2):57–67. https://doi.org/10.1016/3923/je.2012.57.67
Domard A, Domard M (2002) Chitosan structure properties relationship and biomedical applications. Polym Biomater 9:187–212
Erdogan S, Kaya M, Akata I (2017) Chitin extraction and chitosan production from cell wall of two mushroom species (Lactarius vellereus and Phyllophora ribis). AIP Conf Proc 1809(1):020012. https://doi.org/10.1063/1.4975427
Feás X, Vázquez-Tato MP, Seijas JA, Pratima G, Nikalje A, Fraga-López F (2020) Extraction and Physicochemical characterization of Chitin Derived from the asian hornet, Vespa velutina Lepeletier 1836 (Hym.: Vespidae). Molecules 25(2):384. https://doi.org/10.3390/molecules25020384
Gbenebor OP, Adeosun SO, Lawal GI, Jun S, Olaleye SA (2017) Acetylation, crystalline and morphological properties of structural polysaccharide from shrimp exoskeleton. Eng Sci Technol Int J 20(3):1155–1165
Halliday WD, Blouin-Demers G (2014) Red flour beetles balance thermoregulation and food acquisition via density‐dependent habitat selection. J Zool 294(3):198–205
Hu J, Wang W, Dai J, Zhu L (2019) Chemical composition and biological activity against Tribolium castaneum (Coleoptera: Tenebrionidae) of Artemisia brachyloba essential oil. Ind Crops Prod 128:29–37
Imura O, Nakakita H (1984) The effect of temperature and relative humidity on the development of Tribolium freemani Hinton (Coleoptera: Tenebrionidae). J Stored Prod Res 20(2):87–95
Jang MK, Byeong-Gi K, Young-Il J, Chang HL, Jae-Woon N (2004) Physicochemical characterization of α-chitin, β-chitin, and γ-chitin separated from natural resources. J Polym Sci Part A: Polym Chem 42:3423–3432. https://doi.org/10.1002/pola.20176
Juárez-de la Rosa BA, Quintana P, Ardisson PL, Yáñez-Limón JM, Alvarado-Gil JJ (2012) Effects of thermal treatments on the structure of two black coral species chitinous exoskeleton. J Mater Sci 47:990–998. https://doi.org/10.1007/s10853-011-5878-9
Kaya M, Tozak K, Baran T, Sezen G, Sargin I (2013) Natural porous and nano fiber chitin structure from Gammarus argaeus (Gammaridae Crustacea). EXCLI J12:503 – 10
Kaya M, Baublys V, Can E, Šatkauskienė I, Bitim B, Tubelytė V, Baran T (2014) Comparison of physicochemical properties of chitins isolated from an insect (Melolontha melolontha) and a crustacean species (Oniscus asellus). Zoomorph. https://doi.org/10.1007/s00435-014-0227-6
Kaya M, Lelešius E, Nagrockaitė R, Sargin I, Arslan G, Mol A, Baran T, Can E, Bitim B (2015a) Differentiations of chitin content and surface morphologies of chitins extracted from male and female grasshopper species. PLoS ONE 10(1):e0115531. 10.1371/journal.pone.0115531
Kaya M, Mujtaba M, Bulut E, Akyuz B, Zelencova L, Sofi K (2015b) Fluctuation in physicochemical properties of chitins extracted from different body parts of honeybee. Carbohydr Polym 32:9–16. 10.1016/j.carbpol.2015b.06.008
Kaya M, Sofi K, Sargin I, Mujtaba M (2016) Changes in physicochemical properties of chitin at developmental stages (larvae, pupa and adult) of Vespa crabro (wasp). Carbohydr Polym 145:64–70. https://doi.org/10.1016/j.carbpol.2016.03.010
Kaya M, Sargin I, Sabeckis I, Noreikaite D, Erdonmez D, Salaberria AM, Labidi J, Baublys V, Tubelytė V (2017) Biological, mechanical, optical and physicochemical properties of natural chitin films obtained from the dorsal pronotum and the wing of cockroach. Carbohydr Polym 163:162–169. https://doi.org/10.1016/j.carbpol.2017.01.022
Kurita K (2001) Controlled functionalization of the polysaccharide chitin. Prog Polym Sci 26(9):1921–1971
Labandeira CC, Sepkoski JJ (1993) Insect diversity in the fossil record. Science 261(5119):310–315
Laribi-Habchi H, Bouacem K, Allala F, Jabeur F, Selama O, Mechri S, Yahiaoui M, Bouanane-Darenfed A, Jaouadi B (2020) Characterization of chitinase from Shewanella inventionis HE3 with bio-insecticidal effect against granary weevil, Sitophilus granarius Linnaeus (Coleoptera: Curculionidae). Process Biochem 97:222–233. https://doi.org/10.1016/j.procbio.2020.06.023
Liu S, Sun J, Yu L, Zhang C, Bi J, Zhu F, Yang Q (2012a) Extraction and characterization of chitin from the beetle Holotrichia parallela Motschulsky. Molecules 17(4):4604–4611
Liu S, Sun J, Yu L, Zhang C, Bi J, Zhu F, Yang Q (2012b) Extraction and characterization of chitin from the beetle Holotrichia parallela Motschulsky. Molecules 17(4):4604–4611
Mahroof RM, Hagstrum DW, Phillips T, Cuperus GW (2012) In: Cuperus G (ed) Biology, behavior, and ecology of insects in processed commodities. Stored Product Protection Hagstrum, DW, Phillips, TW, pp 33–44
Majtán J, Bíliková K, Markovič O, Gróf J, Kogan G, Šimúth J (2007) Isolation and characterization of chitin from bumblebee (Bombus terrestris). Int J Biol Macromol 40(3):237–241. https://doi.org/10.1016/j.ijbiomac.2006.07.010
Mohammed MH, Williams PA, Tverezovskaya O (2013) Extraction of chitin from prawn shells and conversion to low molecular mass chitosan. Food Hydrocoll 31(2):166–171
Mohan K, Ravichandran S, Muralisankar T, Uthayakumar V, Chandirasekar R, Rajeevgandhi C, Seedevi P (2019) Extraction and characterization of chitin from sea snail Conus inscriptus (Reeve, 1843). Int J Biol Macromol 126:555–560
Mohan K, Ganesan AR, Muralisankar T, Jayakumar R, Sathishkumar P, Uthayakumar V, Chandirasekar R, Revathi N (2020) Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci Technol 105:17–42. https://doi.org/10.1016/j.tifs.2020.08.016
Mohan K, Muralisankar T, Jayakumar R, Rajeevgandhi C (2021) A study on structural comparisons of α-chitin extracted from marine crustacean shell waste. Carbohydr Polym Technol Appl 2:100037. https://doi.org/10.1016/j.carpta.2021.100037
Mohan K, Ganesan AR, Ezhilarasi PN, Kondamareddy KK, Rajan DK, Sathishkumar P, Rajarajeswaran J, Conterno L (2022) Green and eco-friendly approaches for the extraction of chitin and chitosan: a review. Carbohydr Polym 1287:119349. https://doi.org/10.1016/j.carbpol.2022.119349
Mossa ATH (2016) Green pesticides: essential oils as biopesticides in insect-pest management. IJEST 9(5):354
Park BK, Kim MM (2010) Applications of chitin and its derivatives in biological medicine. Int J Mol Sci 11(12):5152–5164
Proespraiwong P, Tassanakajon A, Rimphanitchayakit V (2010) Chitinases from the black tiger shrimp Penaeus monodon: phylogenetics, expression and activities. CBP Part B: Biochemistry and Molecular Biology 156(2):86–96. https://doi.org/10.1016/j.cbpb.2010.02.007
Rees DP (2004) Insects of Stored Products. CSIRO Publishing, Collingwood, Australia
Rinaudo M (2006) Chitin and chitosan: Properties and applications. Prog Polym Sci 31(7):603–632
Rudall KM, Kenchington WJBR (1973) The chitin system. Biol Rev 48(4):597–633
Sajomsang W, Gonil P (2010) Preparation and characterization of α-chitin from cicada sloughs. Mater Sci Eng C 30(3):357–363
Sharbidre A, Sargar S, Gogoi H, Patil R (2021) Characterization of chitin content extracted from edible insect, Coridius nepalensis (Westwood, 1837) (Hemiptera: Dinidoridae). Int J Trop Insect Sci 41:1893–1900. https://doi.org/10.1007/s42690-020-00386-3
Sikorski P, Hori R, Wada M (2009) Revisit of α-chitin crystal structure using high resolution X-ray diffraction data. Biomacromolecules 10(5):1100–1105
Song Z, Li G, Guan F, Liu W (2018) Application of Chitin/Chitosan and their derivatives in the Papermaking Industry. Polymers 10(4):389. https://doi.org/10.3390/polym10040389
Suginta W (2007) Identification of chitin binding proteins and characterization of two chitinase isoforms from Vibrio alginolyticus 283. Enzyme Microb Technol 41(3):212–220
Synowiecki J, Al-Khateeb NA (2003) Production, properties, and some new applications of chitin and its derivatives. Crit Rev Food Sci Nutr 43:145–171
Vetter J (2007) Chitin content of cultivated mushrooms Agaricus bisporus, Pleurotus aotreatus and Lentinula edodes. Food Chem 102:6–9. https://doi.org/10.1016/j.foodchem.2006.01.037
Xu J, McCarthy SP, Gross RA, Kaplan DL (1996) Chitosan film acylation and effects on biodegradability. Macromolecules 29(10):3436–3440
Yen MT, Yang JH, Mau JL (2009) Physicochemical characterization of chitin and chitosan from crab shells. Carbohyd Polym 75:15–21. https://doi.org/10.1016/j.carbpol.2008.06.006
Zhang M, Haga A, Sekiguchi H, Hirano S (2000) Structure of insect chitin isolated from beetle larva cuticle and silkworm (Bombyx mori) pupa exuvia. Int J Biol Macromol 27(1):99–105
Zhang J, Kopparapu NK, Yan Q, Yang S, Jiang Z (2013) Purification and characterisation of a novel chitinase from persimmon (Diospyros kaki) with antifungal activity. Food Chem 138(2–3):1225–1232. https://doi.org/10.1016/j.foodchem.2012.11.067
Zhu Q, Arakane Y, Beeman RW, Kramer KJ, Muthukrishnan S (2008) Characterization of recombinant chitinase-like proteins of Drosophila melanogaster and Tribolium castaneum. Insect Biochem Mol Biol 38(4):467–477. https://doi.org/10.1016/j.ibmb.2007.06.011
Acknowledgements
Authors are thankful to Dr. Kedar Deobhankar, CEO, Dr. Deepak Phal, MD and Mr. Kishor Raut, In-charge of Entomology laboratory of Ross Lifescience Pvt. Ltd., Pune for providing authentic pure cultures of Tribolium castaneum and Sitophilus oryzae.
Funding
This research work is financially supported by UGC- CAS III, Savitribai Phule Pune University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All the authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mistry, A., Sharbidre, A., Patil, R. et al. Properties of chitin isolated from two economically important storage pests, Tribolium castaneum and Sitophilus oryzae. Int J Trop Insect Sci 43, 1401–1409 (2023). https://doi.org/10.1007/s42690-023-01039-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-023-01039-x