Abstract
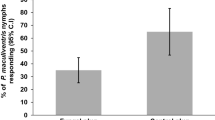
The predatory African weaver ant, Oecophylla longinoda (Hymenoptera: Formicidae) effectively control pests by predation as well as by semiochemicals which influence the behaviour of certain species. Here, we investigated and compared the role of O. longinoda semiochemicals on the oviposition responses of two major fruit fly (Diptera: Tephritidae) species of mango; the invasive species, Bactrocera dorsalis, endemic to Southeast Asia and the African native species, Ceratitis cosyra. We carried oviposition choice assays with ant-exposed and unexposed mangoes, using extracts of ant whole body and body parts. Our results show that the flies avoided and laid fewer eggs on weaver ant-exposed than unexposed mango peel discs. Subsequent assays with mango discs treated with whole body extracts in hexane, ethyl acetate, methanol and water reduced oviposition responses in C. cosyra. Moreover, discs treated with abdominal extracts in hexane and water reduced egg laying in B. dorsalis. whereas hexane, methanol and water extracts of the abdomen reduced oviposition in C. cosyra. Additionally, mango discs treated with extracts of exercised Dufour’s and poison gland in hexane elicited stronger oviposition reduction responses in C. cosyra than in B. dorsalis. These results suggest that ant gland compounds play a role in the oviposition avoidance behaviour of both fruit fly species. Moreover, both African fruit fly species and invasive Asian species, are repelled by Oecophylla longinoda semiochemicals implying that B. dorsalis experienced similar evolutionary pressures, and reacting to similar cues, from Oecophylla smaragdina in Asia as did C. cosyra from Oecophylla longinoda in Africa.










Similar content being viewed by others
References
Adandonon A, Vayssieres JF, Sinzogan A, Van Mele P (2009) Density of pheromone sources of the weaver ant Oecophylla longinoda affects oviposition behaviour and damage by mango fruit flies (Diptera: Tephritidae). Int J Pest Manag 55:285–292
Appiah EF, Ekesi S, Afreh-Nuamah K, Obeng-Ofori D, Mohamed SA (2014) African weaver ant produced semiochemicals impact on foraging behavior and parasitism by the Opiine parasitoid, Fopius arisanus on Bactrocera invadens (Diptera:Tephritidae). Biolog Control 79:49–57
Berendonk UT (1999) Influence of fish kairomones on the ovipositing behavior of Chaoborus imagines. Limnol Oceanogr 44:454–58
Blancafort X, Gomez C (2005) Consequences of the Argentine ant, Linepithema humile (Mayr), invasion on pollination of Euphorbia characias (L.) (Euphorbiaceae). Acta Oecol 28:49–55
Bradshaw JWS, Baker R, Howse PE (1979a) Multicomponent alarm pheromones in the mandibular glands of major workers of the African weaver ant, Oecophylla longinoda. Physiol Entomol 4:15–25
Bradshaw JWS, Baker R, Howse PE (1979b) Chemical composition of the poison apparatus secretions of the African weaver ant, Oecophylla longinoda, and their role in behaviour. Physiol Entomol 4:39–46
Bray A, Nieh J (2014) Non-consumptive predator effects shape honeybee foraging and recruitment dancing. PLoS One 9:e87459
Cornelius ML, Grace JK, Ford PW, Davidson BS (1995) Toxicity and repellency of semiochemicals extracted from a Dolichoderine Ant (Hymenoptera: Formicidae) to the Formosan Subterranean Termite (Isoptera: Rhinotermitidae). Environ Entomol 24:1263–1269
Dicke M, Grostal P (2001) Chemical detection of natural enemies by arthropods: an ecological perspective. Annu. Rev Ecol Syst 32:1–23
Duangphakdee O, Koeniger N, Koeniger G, Wongsiri S, Deowanish S (2005) Reinforcing a barrier- a specific social defense of the dwarf honeybee (Apis florea) released by the weaver ant (Oecophylla smaragdina). Apidologie 36:505–511
Dukas R (2001a) Effects of perceived danger on flower choice by bees. Ecol Lett 4:327–333
Dukas R (2001b) Effects of predation risk on pollinators and plants. In: Chittka L, Thomson J (eds) Cognitive ecology of pollination. Cambridge University Press, Cambridge, pp 214–236
Dukas R (2005) Bumble bee predators reduce pollinator density. Ecol 86:1401–1406
Dukas R, Morse DH (2003) Crab spiders affect flower visitation by bees. Oikos 101:157–163
Ekesi S, Mohamed SA (2011) Mass rearing and quality control parameters for Tephritid fruit flies of economic importance in Africa. In: Akyar I (ed) Wide Spectra of Quality Control. Tech Publishing, Croatia, pp 387–470
Feener DH, Jacobs LF, Schmidt JO (1996) Specialized parasitoid attracted to a pheromone of ants. Anim Behav 51:61–66
Forsgren E (1992) Notes and comments. Am Nat 140:1041–1049
Gonthier DJ (2012) Do herbivores eavesdrop on ant chemical communication to avoid predation? PLoS ONE 7:e28703
Hendel F (1912) H. Sauter's Formosa-Ausbeute. Genus Dacus, Fabricius (1805) (Diptera). Supplementa Entomologica, 1:13–24
Hölldobler B, Wilson EO (1978) Multiple recruitment systems of African weaver ant Oecophylla longinoda (Latreille) (Hymenoptera Formicidae). Behav Ecol Sociobiol 3:19–60
Hooper GHS (1987) Application of quality control procedures for large scale rearing of the Mediterranean fruit fly. Entomol Exp Appl 44:161–167
Keegans SJ, Billen J, Morgan ED (1991) Volatile secretions of the green tree ant Oecophylla Smaragdina (Hymenoptera: Formicidae). Comp Biochem Physiol B: Comp Biochem 100:681–685
Kortet R, Hedrick A (2004) Detection of the spider predator Hololena nedra by naive juvenile field crickets (Gryllus integer) using indirect cues. Behav 141:1189–1196
Latreille PA (1802) Histoire naturelle des fourmis, et recueil de mémoires et d'observations sur les abeilles, les araignées, les faucheurs, et autres insectes. Paris: Impr Crapelet (chez T. Barrois), xvi + 445 pp
Li J, Wang Z, Tan K, Qu Y, Nieh JC (2014) Giant Asian honeybees use olfactory eavesdropping to detect and avoid ant predators. Anim Behav 97:69–76
Maag N, Gehrer L, Woodhams DC (2012) Sink or swim: a test of tadpole behavioral responses to predator cues and potential alarm pheromones from skin secretions. J Comp Physiol (A) 198:841–846
Migani V, Ekesi S, Merkel K, Hoffmeister T (2017) At lunch with a killer: The effect of weaver ants on host-parasitoid interactions on mango. PLoS One 12:e0170101
Oliver TH, Mashanova A, Leather SR, Cook JM, Jansen VAA (2007) Ant semiochemicals limit apterous aphid dispersal. Proc Royal Soc B 274:3127–3131
Oliver TH, Jones I, Cook JM, Leather SR (2008) Avoidance responses of an aphidophagous ladybird, Adalia bipunctata, to aphid-tending ants. Ecol Entomol 33:523–528
Pangle KL, Peacor SD, Johannsson OE (2007) Large nonlethal effects of an invasive invertebrate predator on zooplankton population growth rate. Ecol 88:402–412
Parsons MH, Apfelbach R et al (2017) ‘Biologically meaningful scents’’: a framework for understanding predator–prey research across disciplines.’ Biol Rev 93:98–114
Peng R, Christian K (2004) The weaver ant, Oecophylla smaragdina (Hymenoptera: Formicidae), an effective biological control agent of the red-banded thrips, Selenothrips rubrocinctus (Thysanoptera: Thripidae) in mango crops in the Northern Territory of Australia. Int J Pest Manag 50:107–114
Petranka J, Hayes L (1998) Chemically mediated avoidance of a predatory odonate (Anax junius) by American toad (Bufo americanus) and wood frog (Rana sylvatica) tadpoles. Behav Ecol Sociobiol 42:263–271
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Refsnider JM, Janzen FJ (2010) Putting eggs in one basket: Ecological and evolutionary hypotheses for variation in oviposition-site choice. Annu Rev of Ecol, Evol, and Syst 41:39–57
Renwick JAA, Chew FS (1994) Oviposition behavior in Lepidoptera. Ann Rev Entomol 39:377–400
Romero GQ, Antiqueira PAP, Koricheva J (2011) A Meta-Analysis of predation risk effects on pollinator behaviour. PLoS One 6:e20689
Schwartz NU, Zhong L, Bellemer A, Tracey WD (2012) Egg Laying Decisions in Drosophila are consistent with foraging costs of larval progeny. PLoS One 7:e37910
Sih A (1986) Antipredator responses and the perception of danger by mosquito larvae. Ecol 67:434–441
Singer MS, Rodrigues D, Stireman JO, Carriere Y (2004) Roles of food quality and enemy-free space in host use by a generalist insect herbivore. Ecol 85:2747–2753
Sonan J (1932) Notes on some braconidae and chneumonidae from Formosa, with descriptions of 18 new species. Transactions of the Natural Histori Society of Formosa. Taihoku, 22:66–87
Suttle KB (2003) Ideas and Pollinators as mediators of top-down effects on plants. Ecol Lett 6:688–694
Thompson FC (1998) Fruit fly expert identification system and systematic information database. Journal of the North American Dipterists’ Society, 9, 524
Van Mele P, Vayssières J-F, Van Tellingen E, Vrolijks J (2007) Effects of an African weaver ant, Oecophylla longinoda, in controlling mango fruit flies (Diptera: Tephritidae) in Benin. J Econ Entomol 100(3):695–701
Van Mele P, Vayssieres JF, Adandonon A, Sinzogan A (2009) Ant cues affect the oviposition behaviour of fruit flies (Diptera:Tephritidae) in Africa. Physiol Entomol 34:256–261
Walker F (1849) List of the specimens of dipterous insects in collection of the British Museum, part 4. Br Mus Lond 689 Ð1172
Wennström A, Hjulström LN, Hjältén J, Julkunen-Tiitto R (2010) Mother really knows best: Host choice of adult phytophagous insect females reflects a within-host variation in suitability as larval food. Chemo ecol 20:35–42
Wisenden BD, Chivers DP, Smith RJF (1997) Learned recognition of predation risk by Enallagma damselfly larvae (Odonata: Zygoptera) on the basis of chemical cues. J Chem Ecol 23:137–151
Acknowledgements
The authors are grateful to the German Academic Exchange Service (DAAD) for funding the PhD fellowship, University of Pretoria, and International Centre of Insect Physiology and Ecology (icipe) Capacity Building Program and African Regional Postgraduate Programme in Insect Science (ARPPIS) for hosting the PhD student (BM). Financial support was provided by Agricultural Research for Development (CIRAD) and icipe, core funding provided by UK Aid from the UK Government, Swedish International Development Cooperation Agency (Sida), the Swiss Agency for Development and Cooperation (SDC), Government of the Federal Democratic Republic of Ethiopia, and the Kenyan Government. The views expressed herein do not necessarily reflect the official opinion of the donors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mekonnen, B., Yusuf, A., Pirk, C. et al. Oviposition responses of Bactrocera dorsalis and Ceratitis cosyra to Dufour’s and poison gland extracts of Oecophylla longinoda (Hymenoptera: Formicidae). Int J Trop Insect Sci 41, 2775–2783 (2021). https://doi.org/10.1007/s42690-021-00457-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-021-00457-z