Abstract
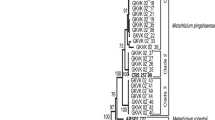
Cowpea aphid, Aphis craccivora Koch is a serious insect pest of cowpea distributed in almost all states of India and its management is essential to increase the quality and quantity of cowpea yield. This study sought to characterize the indigenous isolates of fungal entomopathogens, Beauveria bassiana, Metarhizium pingshaense, M. guizhouense, and Lecanicillium muscarium, using molecular phylogeny and evaluate their pathogenicity against A. craccivora under greenhouse conditions and compare their extracellular chitinase activity. Thirty isolates of fungal entomopathogens were collected from semi-natural and agricultural habitats. Molecular phylogeny of these isolates using partial nuclear sequences, Bloc and MzIGS3, and mitochondrial sequence, nad1, resolved four species. Screening of the isolates against A. craccivora using a single concentration of 1 × 106 conidia/ml revealed that all isolates were pathogenic, with mortality of aphids ranging from 16.67 to 100%. Bioassays of the highly pathogenic isolates of B. bassiana, M. pingshaense, and L. muscarium against A. craccivora revealed low LC50 value of L. muscarium isolate GKVK 03_11 (1.4 × 105 conidia/ml) under greenhouse conditions. Comparison of the extracellular chitinase activity of these isolates detected high chitinase activity of GKVK 03_11 isolate (0.621 U/ml). Study results suggest the biocontrol potential of indigenous L. muscarium isolate for the management of A. craccivora and the possible role of chitinase enzyme in the virulence of fungal entomopathogens.



Similar content being viewed by others
References
Annan IB, Tingey WM, Schaefers GA (1997) Population dynamics and clonal comparisons of Aphis craccivora (Homoptera: Aphididae) on resistant and susceptible cowpea cultivars. Environ Entomol 26:250–255
Bielza P (2008) Insecticide resistance management strategies against the western flower thrips Frankliniella occidentalis. Pest Manag Sci 64:1131–1138
Blackman RL, Eastop VF (2007) Taxonomic issues. In: Van Emden HF, Harringtons R (eds) Aphids as crop pests. CABI Publishing, Wallingford, pp 1–29
Butt TM, Goettel MS (2000) Bioassays of entomogenous fungi. In: Navon A, Ascher KRS (eds) Bioassays of entomopathogenic microbes and nematodes. CABI Publishing, Wallingford, pp 141–195
Charnley AK, St. Leger RJ (1991) The role of cuticle-degrading enzymes in fungal pathogenesis in insects. In: Cole ET, Hoch HC (eds) Fungal spore disease initiation in plants and animals. Plenum Press, New York, pp 267–287
Cito A, Barzanti GP, Strangi A, Francardi V, Zanfini A, Dreassi E (2016) Cuticle-degrading proteases and toxins as virulence markers of Beauveria bassiana (Balsamo) Vuillemin. J Basic Microbiol 56:941–948
Cullings KW (1992) Design and testing of a plant specific PCR primer for ecological and evolutionary studies. Mol Ecol 1:233–240
Gao Y, Lei Z, Reitz SR (2012) Western flower thrips resistance to insecticides: detection, mechanisms and management strategies. Pest Manag Sci 68:1111–1121
Goettel MS, St Leger RJ, Rizzo NW, Staples RC, Roberts DW (1989) Ultrastructural localization of a cuticle-degrading protease produced by the entomopathogenic fungus Metarhizium anisopliae during penetration of host (Manduca sexta). J Gen Microbiol 135:2233–2239
Hajek AE, St. Leger RJ (1994) Interactions between fungal pathogens and insect hosts. Annu Rev Entomol 39:293–322
Herselman L, Thwaites R, Kimmins FM, Courtois B, Van Der Merwe PJA, Seal SE (2004) Identification and mapping of AFLP markers linked to peanut (Arachis hypogaea L.) resistance to the aphid vector of groundnut rosette disease. Theor Appl Genet 109:1426–1433
Hoffmann MP, Frodsham AC (1993) Natural Enemies of Vegetable Insect Pests. Cooperative Extension, Cornell University, Ithaca, 63 pp
Imoulan A, Hussain M, Kirk PM, Meziane AE, Yao YJ (2017) Entomopathogenic fungus Beauveria: host specificity, ecology and significance of morpho-molecular characterization in accurate taxonomic classification. J Asia Pac Entomol 20:1204–1212
Kataria R, Kumar D (2017) Studies on the presence of Semiochemicals E-β-Farnesene (EBF) from Aphis craccivora (Koch), Gujarat, India. J Entomol Zool Stud 5:120–129
Kepler RM, Rehner SA (2013) Genome-assisted development of nuclear intergenic sequence markers for entomopathogenic fungi of the Metarhizium anisopliae species complex. Mol Ecol Resour 13:210–217
Kouvelis VN, Sialakouma A, Typas MA (2008) Mitochondrial gene sequences alone or combined with ITS region sequences provide firm molecular criteria for the classification of Lecanicillium species. Mycol Res 112:829–844
Latgé JP, Papierok B (1988) Aphid pathogens. In: Minks AK, Harrewijn P (eds) Aphids - their biology, natural enemies and control, vol 2B. Elsevier, Amsterdam, pp 323–335
LeOra Software (1987) POLO-PC a user’s guide to probit or logit analysis. LeOra Software, Berkeley
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Mohammed AA, Kadhim JH, Kamaluddin ZNA (2018) Selection of highly virulent entomopathogenic fungal isolates to control the greenhouse aphid species in Iraq. Egypt J Biol Pest Co 28:71
Ofuya TI (1997) Control of the cowpea aphid, Aphis craccivora Koch (Homoptera: Aphididae), in cowpea Vigna unguiculata (L.) Walp. Integr Pest Manag Rev 2:199–207
Patil S, Sridevi D, Babu TR, Pushpavathi B (2017) Relative efficacy of selected insecticides on cowpea aphid, Aphis craccivora (Koch). J Entomol Zool Stud 5:1603–1607
Ramanujam B, Poornesha B, Dileep RC, Japur K (2017) Field evaluation of entomofungal pathogens against cowpea aphid, Aphis craccivora Koch and their effect on two coccinellid predators. Int J Pest Manag 63:101–104
Rehner SA, Posada F, Buckley EP, Infante F, Castillo A, Vega FE (2006) Phylogenetic origins of African and Neotropical Beauveria bassiana pathogens of the coffee berry borer, Hypothenemus hampei. J Invertebr Pathol 93:11–21
Silvestro D, Michalak I (2012) raxmlGUI: a graphical front-end for RAxML. Org Divers Evol 12:335–337
Singh SR, Jackai LEN (1985) Insect pests of cowpeas in Africa: their life cycle, economic importance and potential for control. In: Singh SR, Rachies KO (eds) Cowpea research, production and utilization. Wiley, Chichester, pp 217–231
SPSS Inc (2008) SPSS statistics for windows, version 17.0. SPSS Inc, Chicago
St. Leger RJ, Cooper RM, Charnley AK (1986) Cuticle-degrading enzymes of entomopathogenic fungi: cuticle degradation in vitro by enzymes from entomopathogens. J Invertebr Pathol 47:167–177
Suresh BC, Khan HK, Prasanna PM (2012) Efficacy of different entomopathogenic fungi against cowpea aphid, Aphis craccivora Koch under laboratory and field condition. Int J Plant Prot 5:68–71
Zimmermann G (1986) The ‘Galleria bait method’ for detection of entomopathogenic fungi in soil. J Appl Entomol 102:213–215
Acknowledgements
I thank Science and Engineering Research Board (SERB) Start-up grant for Young Scientists scheme (SR/FT/LS-156/2010), Govt. of India, New Delhi for providing financial support of this work. I am grateful to advisor late Dr. C. K. Suresh for his guidance in the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has declared no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Table S1
Fungal entomopathogens collected with their GPS coordinates and locality, host/ substratum, GenBank accession number and mean per cent mortality of Aphis craccivora against isolates using concentration of 1 × 106 conidia/ml (DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Juliya, R.F. Phylogeny, chitinase activity, and pathogenicity of Beauveria, Metarhizium and Lecanicillium species against cowpea aphid, Aphis craccivora Koch. Int J Trop Insect Sci 40, 309–314 (2020). https://doi.org/10.1007/s42690-019-00082-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-019-00082-x