Abstract
Introduction
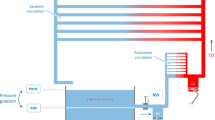
Several models of venous occlusion plethysmography (VOP) aim to detect venous mechanical dysfunctions. However, such models ignore arterial contribution to plethysmographic signal. Arterial resistance is an important factor because it regulates blood inflow through veins. Furthermore, modeling arterial compliance and resistance permits evaluation of arterial pressure throughout VOP protocol.
Method
This study presents a new simulation model and VOP data interpretation, making it possible to estimate arterial and venous mechanical characteristics. The model was evaluated using VOP data obtained under different vascular conditions (placebo, post-ibuprofen, and post-exercise).
Results
Estimated mean values of venous compliance were 0.34 ml/mmHg (placebo), 0.33 ml/mmHg (post-ibuprofen), and 0.27 ml/mmHg (post-exercise). The corresponding estimated values of venous resistance were 11.55 mmHg s/ml (placebo), 10.58 mmHg s/ml (post-ibuprofen), and 8.84 mmHg s/ml (post-exercise). The estimated mean values of arteriolar resistance were 132.14 mmHg s/ml (placebo), 123.93 mmHg s/ml (post-ibuprofen), and 95.36 mmHg s/ml (post-exercise).
Conclusion
The estimated values are consistent with previous VOP model results and with expected physiological behavior. The proposed model can provide further information for studies using the VOP technique including studies involving exercise, reactive hyperemia, mental stress, body temperature changes, and vasomotor substance administration.






Similar content being viewed by others
References
Anderson FA Jr, Durgin WW, Wheeler HB. Interpretation of venous occlusion plethysmography using a nonlinear model. Med Biol Eng Comput. 1986;24:379–85.
Anderson EA, Mark AL. Flow-mediated and reflex changes in large peripheral artery tone in humans. Circulation. 1989;79:93–100.
Choudhury AD. Benerjee R. Sinha A. Kundu S. Estimating blood pressure using wiindkessel modelo n photoplethysmogram. Conf Proc IEEE Eng Med Biol Soc 2014:4567–70.
Daiber A, Chlopicki S. Revisiting pharmacology of oxidative stress and endothelial dysfunction in cardiovascular disease: evidence for redox-based therapies. Free Radic Biol Med. 2020. In press;157:15–37.
de Logu F, Puma SL, Landini L, Tuccinardi T, Poli G, Preti D, et al. The acyl-glucuronide metabolite of ibuprofen has analgesic and anti-inflammatory effects via the TRPA1 channel. Pharmacol Res. 2019;142:127–39.
Eiken O, Kölegard R. Comparation of vascular distensibility in the upper and lower extremity. Acta Physiol Scand. 2004;181:281–7.
Fokunang CN, Fokunang ET, Frederick K, Ngameni B, Ngadjui B. Overview of non-steroidal anti-inflammatory drugs (nsaids) in resource limited countries. MOJ Toxicol. 2018;4(1):5–13.
Hall JE. Guyton and Hall textbook of medical physiology. 1st ed. Amman: Elsevier; 2016.
Hoeks APG, Samijo SK, Brands PJ, Reneman RS. Noninvasive determination of shear-rate distribution across the arterial lumen. Hypertension. 1995;26:26–33.
Hokanson DE. EC6 plethysmograph manual. EUA: Hokanson; 1998.
Jonhson SA, Litwin NS, Seals DR. Age-related vascular dysfunction: what registered dietitian nutritionists need to know. J Acad Nutr Diet. 2019;119(11):1785–96.
Junejo RT, Ray CJ, Marshall JM. Cuff inflation time significantly affects blood flow recorded with venous occlusion plethysmography. Eur J Appl Physiol. 2019;119:665–74.
Kim WJ, Kim JW, Moon YJ, Kim SH, Hwang GS, Shin WJ. The photoplethysmographic amplitude to pulse pressure ratio can track sudden changes in vascular compliance and resistance during liver graft reperfusion: a beat-to-beat analysis. Medicine. 2017;96(22):e7045.
Kundu RN, Biswas S, Das M. Mean arterial pressure classification: a better toll for statistical interpretation of blood pressure related risk covariates. Cardiol Angiol. 2017;6(1):1–7.
Lee J. Noh HJ. Yoon YR. Yoon HR. Lee KJ. Mathematical model of venous occlusion plethysmography for diagnosing deep vein thrombosis. Proceedings of the 23rd Annual EMBS International Conference. Istanbul; Turkey. 2001:25–8.
Macedo AR. Nobrega, ACL, Souza MN. Blood flow dynamics characteristics based on plethysmographic measurements. XXIV Congress of the International Society of Biomechanics. XV Brazilian Congress of Biomechanics. Porto de Galinhas; 2013.
Macedo AR, Nobrega ACL, Machado JC, Souza MN. Assessment of characteristic of the vasomotor control dynamics based on plethysmographic blood flow measurement. Physiol Meas. 2008;29:205–15.
Magder S. Volume and its relationship to cardiac output and venous return. Critical Care. 2016;20(1):271–81.
Margaritelis NV, Paschalis V, Theodorou AA, Kyparos A, Nikolaidis MG. Redox basis of exercise physiology. Redox Biol. 2020;35:101499. https://doi.org/10.1016/j.redox.2020.101499.
Naylor HL, Shoemaker JK, Bock RW, Hughson RL. Prostaglandin inhibition causes an increase in reactive hyperemia after ischemia exercise in human forearm. Clin Physiol. 1999;19:211–20.
Nejad E, Carey JP, McMurtry MS, Hahn JO. Model-based cardiovascular disease diagnosis: a preliminary in-silico study. Biomech Model Mochano Biol. 2017;16(2):549–60.
Olver D, Reid SM, Smith AR, Zamir M, Lemon PWR, Laughlin MH, et al. Effects of acute and chronic interval sprint exercise performed on manually propelled treadmill on upper limb vascular mechanics in health young men. Phys Rep. 2016;4(3):e12861.
Oue A, Sato K, Yoneya M, Sadamoto T. Decreased compliance in the deep and superficial conduit veins of the upper arm during prolonged cycling exercise. Plysiol Reports. 2017;5(8):e13253.
Oue A, Saito M, Iimura Y. Effect of short-term endurance training on venous compliance in the calf and forearm differs between continuous and interval exercise in humans. Phys Rep. 2019;7(17):e14211.
Park KH, Park WJ. Endothelial dysfunction: clinical implications in cardiovascular disease and therapeutic approaches. J Korean Med Sci. 2015;30:1213–25.
Peng LP, Wen J, Yang K, Zhao SL, Dai J, Liang ZS, et al. Effects of arterial blood on the venous blood vessel wall and differences in percentages of lymphocytes and neutrophils between arterial and venous blood. Medicine. 2018;97(26):e11201.
Pescatello L, Fargo AE, Leach CN, Scherzer HH. Short-term effect on dynamic exercise on arterial blood pressure. Circulation. 2014;83(5):1557–61.
Planken RN, Keuter XH, Kessels AG, Hoeks AP, Leiner T, Tordoir JH. Forearm cephalic vein cross-sectional area changes at incremental congestion pressures: towards a standardized and reproducible vein mapping protocol. J Vasc Surg. 2006;44:353–8.
Roelofs E, Smith-Ryan A, Trexler ET, Hirsch KR, Mock MG. Effects of pomegranate extract on blood flow and vessel diameter after high-intensity exercise in young, health adults. Eur J Sport Sci. 2017;17(3):317–25.
Ruchti TL. Brown RH. Feng X. Jeutter DC. Estimation of systemic arterial parameters for control of an electrically actuated total artificial heart. IEE Circuits and Systems Proceedings of the 32nd Midwest Symposium. Champaign. IL; USA. 1989: 640–3.
Salisbury D, Brown RJL, Bronas UG, Kirk LN, Treat-Jacobson D. Measurement of peripheral blood flow in patients with peripheral artery disease: methods and considerations. Vasc Med. 2018;23(2):163–71.
Sandblom E, Axelsson M, McKenzie DJ. Venous responses during exercise in rainbow trout. Oncorhynchus mykiss: alpha-adrenergic control and antihypotensive function of the renin-angiotensin system. Comp Biochem Phys Part A. 2006;144:401–9.
Seagar AD, Gibbs JM, Davis FM. Interpretation of venous occlusion plethysmography measurements using a simple model. Med Biol Eng Comput. 1984;22:12–8.
Shi Y, Lawford P, Hose DR. Construction of a lumped-parameter cardiovascular models using the CellML language. J Med Eng Technol. 2018;42(7):525–31.
Shimizu S, Une D, Kawada T, Hayama Y, Kamiya A, Shishido T, et al. Lumped parameter model for hemodynamic simulation of congenital heart disease. J Physiol Sci. 2018;68(2):103–11.
Silva BM, Neves FJ, Rocha NG, Cagy M, Souza MN, Nóbrega ACL. Intra- and inter-tester reproducibility of venous occlusion plethysmography: comparison between a manual and a semi-automatic method of blood flow analysis. Physiol Meas. 2009;30:1267–79.
Sinton AM, Seagar AD. Automated venous occlusion plethysmography. Med Biol Eng Comput. 1988;26:295–302.
Skoog J, Zachrisson H, Lindenberger M, Ekman M, Ewerman L, Länne T. Calf venous compliance measured by venous occlusion plethysmography: methodological aspects. Eur J Appl Physiol. 2015;115:245–56.
Storch AS, Mattos JD, Alves R, Galdino IS, Rocha HNM. Melhods of endothelial function assessment: description and applications. Int J Cardiovasc Sci. 2017;30(3):262–73.
Tansey EA, Montgomery LEA, Quinn JG, Roe SM, Johnson CD. Understanding basic vein physiology and venous blood pressure through simple physical assessment. Adv Physiol Educ. 2019;43:423–9.
Turner IC, McNally MA, O’Connell BM, Cooke EA, Kernohan WG, Mollan RAB. Numerical model of deep venous thrombosis detection using venous occlusion plethysmography. Med Biol Eng Comput. 2000;38:348–55.
Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Ciková R, Cosentino F, et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from to European Society of Cardiology working group on peripheral circulation endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis. 2015;241:507–32.
Acknowledgments
This research was supported by the Brazilian governmental agencies: the National Counsel of Technological and Scientific Development (CNPq) and the Coordination for the Improvement of Higher Level or Education-Personnel (CAPES).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Macedo, A.R., Machado, J.C., Luz, L.M.S. et al. A novel model to simulate venous occlusion plethysmography data and to estimate arterial and venous parameters. Res. Biomed. Eng. 36, 463–473 (2020). https://doi.org/10.1007/s42600-020-00087-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42600-020-00087-3