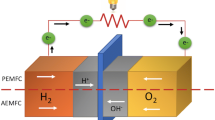
Abstract
Chitosan is considered one of the most promising materials for polymer electrolyte membrane fuel cells (PEMFC) due to the high manufacturing cost of the commercial polymer electrolyte membrane (PEM). In this study, chitosan (C-1), chloroacetate chitosan (CCS-21), epoxidized natural rubber (ENR-41), chitosan blended with ENR (CE-6 and CE-7), and chloroacetate chitosan blended with ENR (CCE-26 and CCE-27) based membranes were prepared by solution casting technique and crosslinked with acid and base. The Fourier transform infrared (FTIR) spectroscopy results showed improvements in their peaks, and the atomic force microscopy revealed that the blended membranes had a higher surface roughness than the other membranes. The optical contact angle (OCA) showed that the C-1 had the highest contact angle, while the ENR-41 had the least contact angle. From the electrical measurements, the CCS-21 gave the highest capacitance, conductance, and conductivity. The CE-7 showed the highest resistance and resistivity while ENR-41 showed the highest dissipation factor and dielectric constant. Taken together, CE-7 showed the most suitable characteristics which makes it suitable for use as a PEM.











Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Wakihara M (2001) Recent developments in lithium ion batteries. Mater Sci Eng R 33(4):109–134. https://doi.org/10.1016/S0927-796X(01)00030-4
Appleby AJ, Foulkes FR (1989) Fuel cell handbook. Van Nostrand Reinhold, New York
Smitha B, Sridhar S, Khan AA (2006) Chitosan–poly(vinyl pyrrolidone) blends as membranes for direct methanol fuel cell applications. J Power Sources 159(2):846–854. https://doi.org/10.1016/j.jpowsour.2005.12.032
Heinzel A, Barragán VM (1999) A review of the state-of-the-art of the methanol crossover in direct methanol fuel cells. J Power Sources 84(1):70–74. https://doi.org/10.1016/S0378-7753(99)00302-X
Kamarudin SK, Achmad F, Daud WRW (2009) Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. Int J Hydrogen Energy 34(16):6902–6916. https://doi.org/10.1016/j.ijhydene.2009.06.013
Beattie PD, Orfino FP, Basura VI, Zychowska K, Ding J, Chuy C, Schmeisser J, Holdcroft S (2001) Ionic conductivity of proton exchange membranes. J Electroanal Chem 503(1–2):45–56. https://doi.org/10.1016/S0022-0728(01)00355-2
Hasani-Sadrabadi MM, Dorri NM, Ghaffarian SR, Dashtimoghadam E, Sarikhani K, Majedi FS (2010) Effects of organically modified nanoclay on the transport properties and electrochemical performance of acid-doped polybenzimidazole membranes. J Appl Polym Sci 117(2):1227–1233. https://doi.org/10.1002/app.31974
Blair HS, Ho T-C (2007) Studies in the adsorption and diffusion of ions in chitosan. J Chem Technol Biotechnol 31(1):6–10. https://doi.org/10.1002/jctb.503310103
Cui Z, Xiang Y, Si J, Yang M, Zhang Q, Zhang T (2008) Ionic interactions between sulfuric acid and chitosan membranes. Carbohyd Polym 73(1):111–116. https://doi.org/10.1016/j.carbpol.2007.11.009
Krajewska B, Olech A (1996) Pore structure of gel chitosan membranes. I. Solute diffusion measurements. Polym Gels Netw 4(1):33–43. https://doi.org/10.1016/0966-7822(95)00015-1
Wan Y, Creber KAM, Peppley B, Bui VT (2003) Ionic conductivity of chitosan membranes. Polymer 44(4):1057–1065. https://doi.org/10.1016/S0032-3861(02)00881-9
Niamsa N, Baimark Y (2009) Preparation and characterization of highly flexible chitosan films for use as food packaging. Am J Food Technol 4(4):162–169. https://doi.org/10.3923/ajft.2009.162.169
Krajewska B (2001) Diffusional properties of chitosan hydrogel membranes. J Chem Technol Biotechnol 76(6):636–642. https://doi.org/10.1002/jctb.429
Matsuyama H, Kitamura Y, Naramura Y (1999) Diffusive permeability of ionic solutes in charged chitosan membrane. J Appl Polym Sci 72(3):397–404. https://doi.org/10.1002/(SICI)1097-4628(19990418)72:3%3c397::AID-APP9%3e3.0.CO;2-C
Wang XP, Shen ZQ, Zhang FY, Zhang YF (1996) A novel composite chitosan membrane for the separation of alcohol-water mixtures. J Membr Sci 119(2):191–198. https://doi.org/10.1016/0376-7388(96)00157-3
Wei YC, Hudson SM, Mayer JM, Kaplan DL (1992) The crosslinking of chitosan fibers. J Polym Sci Part A 30(10):2187–2193. https://doi.org/10.1002/pola.1992.080301013
Wan Y, Creber KAM, Peppley B, Bui VT (2003) Ionic conductivity and related properties of crosslinked chitosan membranes. J Appl Polym Sci 89(2):306–317. https://doi.org/10.1002/app.12090
Wan Y, Peppley B, Creber KAM, Bui VT (2010) Anion-exchange membranes composed of quaternized-chitosan derivatives for alkaline fuel cells. J Power Sources 195(12):3785–3793. https://doi.org/10.1016/j.jpowsour.2009.11.123
Ali Q, Taweepreda W, Techato K (2020) Preparation and characterization of polymer electrolyte membrane from chloroacetate chitosan/chitosan blended with epoxidized natural rubber. Polym Testing 82:106294. https://doi.org/10.1016/j.polymertesting.2019.106294
Baker CSL, Gelling IR, Newell R (1985) Epoxidized natural rubber. Rubber Chem Technol 58(1):67–85. https://doi.org/10.5254/1.3536059
Ismail H, Shaari SM, Othman N (2011) The effect of chitosan loading on the curing characteristics, mechanical and morphological properties of chitosan-filled natural rubber (NR), epoxidised natural rubber (ENR) and styrene-butadiene rubber (SBR) compounds. Polym Testing 30(7):784–790. https://doi.org/10.1016/j.polymertesting.2011.07.003
Yoksan R (2008) Epoxidized natural rubber for adhesive applications. Nat Sci 42(5):325–332. https://doi.org/10.1007/978-3-642-14094-5
Idris R, Glasse MD, Latham RJ, Linford RG, Schlindwein WS (2001) Polymer electrolytes based on modified natural rubber for use in rechargeable lithium batteries. J Power Sources 94(2):206–211. https://doi.org/10.1016/S0378-7753(00)00588-7
Raju G, Mas Haris MRH (2016) Preparation and characterization of acidified chitosan immobilized in epoxidized natural rubber. Polym Testing 53:1–6. https://doi.org/10.1016/j.polymertesting.2016.05.005
Ahmed F, Kumar S, Arshi N, Anwar MS, Su-Yeon L, Kil GS, Park DW, Koo BH, Lee CG (2011) Preparation and characterizations of polyaniline (PANI)/ZnO nanocomposites film using solution casting method. Thin Solid Films 519(23):8375–8378. https://doi.org/10.1016/j.tsf.2011.03.090
Mariani E, Eckstein J, Rubínová E (1967) Permittivity, dielectric loss and dc conductivity of NaNO3 crystals grown by epitaxy. Czech J Phys 17(6):552–558. https://doi.org/10.1007/BF01695178
Edmondson CA, Stallworth PE, Wintersgill MC, Fontanella JJ, Dai Y, Greenbaum SG (1998) Electrical conductivity and NMR studies of methanol/water mixtures in Na ® on membranes. Electrochimica Acta 43(10–11):1295–1299. https://doi.org/10.1016/S0013-4686(97)10033-0
Taweepreda W, Wichianchom A (2013) Fabrication and characterization of epoxidized natural rubber-chitosan membrane. Adv Mater Res 844:205–208. https://doi.org/10.4028/www.scientific.net/amr.844.205
Wanichapichart P, Sungkum R, Taweepreda W, Nisoa M (2009) Characteristics of chitosan membranes modified by argon plasmas. Surf Coat Technol 203(17–18):2531–2535. https://doi.org/10.1016/j.surfcoat.2009.02.069
Smitha B, Sridhar S, Khan AA (2005) Chitosan-sodium alginate polyion complexes as fuel cell membranes. Eur Polymer J 41(8):1859–1866. https://doi.org/10.1016/j.eurpolymj.2005.02.018
Balau L, Lisa G, Popa MI, Tura V, Melnig V (2004) Physico-chemical properties of chitosan films. Cent Eur J Chem 2(4):638–647. https://doi.org/10.2478/BF02482727
Cho SA, Cho EA, Oh IH, Kim HJ, Ha HY, Hong SA, Ju JB (2006) Surface modified Nafion® membrane by ion beam bombardment for fuel cell applications. J Power Sources 155(2):286–290. https://doi.org/10.1097/01.mcg.0000210103.82241.97
De Oliveira RRL, Albuquerque DAC, Cruz TGS, Yamaji FM, Leite FL (2012) Measurement of the nanoscale roughness by atomic force microscopy: basic principles and applications [Internet]. In: Atomic force microscopy—imaging, measuring and manipulating surfaces at the atomic scale. InTech, London. https://doi.org/10.5772/37583
Lehmani A, Durand-Vidal S, Turq P (1998) Surface morphology of nafion 117 membrane by tapping mode atomic force microscope. J Appl Polym Sci 68(3):503–508. https://doi.org/10.1002/(SICI)1097-4628(19980418)68:3%3c503::AID-APP16%3e3.0.CO;2-V
Liu J, Wang H, Cheng S, Chan KY (2005) Nafion-polyfurfuryl alcohol nanocomposite membranes for direct methanol fuel cells. J Membr Sci 246(1):95–101. https://doi.org/10.1016/j.memsci.2004.08.016
Rankl M, Laib S, Seeger S (2003) Surface tension properties of surface-coatings for application in biodiagnostics determined by contact angle measurements. Coll Surf B 30(3):177–186. https://doi.org/10.1016/S0927-7765(03)00085-7
Zawodzinski TA, Gottesfeld S, Shoichet S, McCarthy TJ (1993) The contact angle between water and the surface of perfluorosulphonic acid membranes. J Appl Electrochem 23(1):86–88. https://doi.org/10.1007/BF00241582
Bass M, Berman A, Singh A, Konovalov O, Freger V (2010) Surface structure of nafion in vapor and liquid. J Phys Chem B 114(11):3784–3790. https://doi.org/10.1021/jp9113128
Goswami S, Klaus S, Benziger J (2008) Wetting and absorption of water drops on nafion films. Langmuir 51:8627–8633. https://doi.org/10.1021/la800799a
Moilanen DE, Piletic IR, Fayer MD (2007) Water dynamics in nafion fuel cell membranes: the effects of confinement and structural changes on the hydrogen bond network. J Phys Chem C 111(25):8884–8891. https://doi.org/10.1021/jp067460k
Baustista-Rodríguez CM, Rosas-Paleta A, Rivera-Márquez JA, Solorza-Feria O (2009) Study of electrical resistance on the surface of nafion 115® membrane used as electrolyte in PEMFC technology part I: Statistical inference. Int J Electrochem Sci 4(1):43–59
Fontanella JJ, Edmondson CA, Wintersgill MC, Wu Y, Greenbaum SG (1996) High-pressure electrical conductivity and NMR studies in variable equivalent weight Nafion membranes. Macromolecules 29(14):4944–4951. https://doi.org/10.1021/ma9600926
Zaidi SMJ (2005) Preparation and characterization of composite membranes using blends of SPEEK/PBI with boron phosphate. Electrochim Acta 50(24):4771–4777. https://doi.org/10.1016/j.electacta.2005.02.027
Opekar F, Svozil D (1995) Electric resistance in a Nafion® membrane exposed to air after a step change in the relative humidity. J Electroanal Chem 385(2):269–271. https://doi.org/10.1016/0022-0728(95)03767-B
Dweiri R, Sahari J (2007) Electrical properties of carbon-based polypropylene composites for bipolar plates in polymer electrolyte membrane fuel cell (PEMFC). J Power Sources 171(2):424–432. https://doi.org/10.1016/j.jpowsour.2007.05.106
Yusmaniar Y, Afrizal A, Nurzaman N, Handoko E (2018) Structure and electrical properties of polymer electrolyte membrane for fuel cell application. MATEC Web Conf 197(12 September 2018):04003. https://doi.org/10.1051/matecconf/201819704003
Lu Z, Lanagan M, Manias E, Macdonald D (2010) Dielectric properties of polymer electrolyte membranes measured by two-port transmission line technique. Electrochem Soc 28(29):95–105. https://doi.org/10.1149/1.3502448
Gimba ID, Abdulkareem AS, Jimoh A, Afolabi AS (2016) Theoretical energy and exergy analyses of proton exchange membrane fuel cell by computer simulation. J Appl Chem. https://doi.org/10.1155/2016/2684919
Lu Z, Polizos G, Macdonald DD, Manias E (2008) State of water in perfluorosulfonic ionomer (Nafion 117) proton exchange membranes. J Electrochem Soc 155(2):B163. https://doi.org/10.1149/1.2815444
Taweepreda W (2014) Dynamic mechanical and dielectric properties of modified surface chitosan/natural rubber latex. Sains Malaysiana 43(2):241–245
Acknowledgements
AW would like to thank the Thai Government Scholarship for Ph.D. studentship and grateful to thank Rajamangala University of Technology Srivijaya, Nakhon Si Thammarat Saiyai Campus and Faculty of Science, Prince of Songkla University for others supporting.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wichianchom, A., Taweepreda, W., Ali, Q. et al. Modification of chitosan-based membrane with epoxidized natural rubber for PEMFC application. J Rubber Res 26, 233–247 (2023). https://doi.org/10.1007/s42464-023-00215-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42464-023-00215-5