Abstract
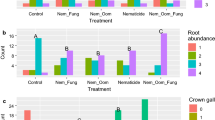
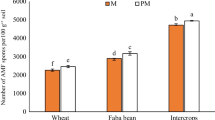
The rubber tree (Hevea brasiliensis)–cassava (Manihot esculenta) intercropping system is one of the most popular intercropping patterns for small-holder rubber planters. However, this practice is not recommended in some rubber-producing countries, since it is believed that the rubber tree and cassava share some identical pathogens and that the intercropping system will deplete soil fertility. The effects of cassava allelochemicals on rubber tree pathogens have never been reported. Using controlled plate tests, pot tests, and field experiments, this study aimed to assess the possible adverse effects that cassavas may impart to rubber plantations. The results showed: (1) although the leaf extract, decomposed leaf extract and root exudates of cassava inhibited or had no effect on the mycelial growth of the rubber tree pathogen Corynespora cassiicola, 10 mg mL−1 and 100 mg mL−1 cassava leaf water extracts and testing concentrations of cassava root extraction significantly promoted the mycelial growth of the rubber tree pathogen Rigidoporus lignosus, which increased the risk of rubber tree white root disease; (2) the overall soil fertility was increased significantly in the rubber tree–cassava intercropping system. However, the soil phosphorus content was reduced significantly in the field experiment; (3) the average girth of rubber trees was significantly reduced, and the girth uniformity was decreased in the intercropping system. Based on the experiment results, we suggested that an optimised fertiliser recommendation, particularly on the phosphorus amount, should be developed and implemented for the rubber tree–cassava intercropping system. In addition, since the intercropping of cassava will increase the risk of R. lignosus-induced rubber tree white root disease, which is very hard to prevent and eradicate once rubber trees are infected, the intercropping of cassava in rubber plantation rows should not be recommended in the R. lignosus prevailing areas.



Similar content being viewed by others
References
Chen S, Peng S, Huang G, Wu K, Fu X, Chen Z (2003) Association of decreased expression of a MYB transcription factor with the TPD (tapping panel dryness) syndrome in Hevea brasiliensis. Plant Mol Biol 51(1):51–58
Mo Y (2014) Natural rubber is produced in more than 60 countries in the world. China Trop Agric 5:75–76
Mo Y, Yang L (2018) Domestic and oversea natural rubber industry development situation in 2017. World Trop Agric Inf 2:1–3
Clermont-Dauphin C, Dissataporn C, Suvannang N, Pongwichian P, Maeght J-L, Hammecker C, Jourdan C (2018) Intercrops improve the drought resistance of young rubber trees. Agron Sustain Dev 38(6):56
Esekhade T, Orimoloye J, Ugwa I, Idoko S (2003) Potentials of multiple cropping systems in young rubber plantations. J Sustain Agric 22(4):79–94
Li J, Zhou L, Lin W (2019) Calla lily intercropping in rubber tree plantations changes the nutrient content, microbial abundance, and enzyme activity of both rhizosphere and non-rhizosphere soil and Calla lily growth. Ind Crop Prod 132:344–351
Chen C, Liu W, Wu J, Jiang X, Zhu XJG (2019) Can intercropping with the cash crop help improve the soil physico-chemical properties of rubber plantations? Geoderma 335:149–160
Langenberger G, Cadisch G, Martin K, Min S, Waibel H (2017) Rubber intercropping: a viable concept for the 21st century? Agrofor Syst 91(3):577–596
Rodrigo V, Stirling C, Silva T, Pathirana P (2005) The growth and yield of rubber at maturity is improved by intercropping with banana during the early stage of rubber cultivation. Field Crop Res 91(1):23–33
Lin W, Zhen F, Li J, Li Z, Li C, Chen Y, Yan Z, Fu J, Xie G, An F, Wang J, Zhou J (2016) Regulation of rubber tree cultivation [S]. Ny/T221-2016. Ministry of China, Beijing
Paisan L (1996) Intercropping of young rubber. Suranaree J Sci Technol 3:171–179
Li J, Zhou L, Lin W (2018) Competitive characteristics related to nitrogen utilization and calla lily growth in rubber-calla lily intercropping systems. Ind Crop Prod 125:567–572
Huang J, Zhou J, Lin S, Chen Y, Liu X, Li X (2014) Thinking and expectation on intercropping cassava in rubber plantation. Acta Agric Jiangxi 26(1):64–71
Esekhade TU, Idoko SO, Osazuwa Okore IK, Mesike CS (2014) Effect of intercropping on the gestation period of rubber. Wudpecker J Agric Res 3(8):150–153
Cai J, Chen Y, Pan X, Huang G (2008) Disease severity investigation and pathogen identification of corynespora leaf fall disease of Heava brasiliensis in Hainan province. Chin J Trop Agric 28(05):1–7
Yang G (2006) Rubber-grain intercropping system in Gabo. World Trop Agric Inf 6:1–2
Bertin C, Yang X, Weston LA (2003) The role of root exudates and allelochemicals in the rhizosphere. Plant Soil Tillage Res 256(1):67–83
Narwal SS, Hoagland RE, Dilday RH, Roger MR (2000) Allelopathy in ecological agriculture and forestry. Springer Science and Business Media, Berlin
Singh H, Batish DR, Kohli R (2001) Allelopathy in agroecosystems: an overview. J Crop Prod 4(2):1–41
Devi M (2017) Allelopathy in agroforestry: a review. J Pharmacogn Phytochem 6(3):686
Vivanco JM, Baluška F (2012) Secretions and exudates in biological systems. Springer Science and Business Media, New York
Zeng RS, Mallik AU, Luo SM (2008) Allelopathy in sustainable agriculture and forestry. Springer, New York
Cereda M, Mattos M (1996) Linamarin: the toxic compound of cassava. J Venom Anim Toxins 2(1):06–12
Ferraro V, Piccirillo C, Tomlins K, Pintado ME (2016) Cassava (Manihot esculenta Crantz) and Yam (Dioscorea spp.) crops and their derived foodstuffs: safety, security and nutritional value. Crit Rev Food Sci Nutr 56(16):2714–2727
He CW, Chen YP, Qin JP, Chen ZY (2011) Preliminary tests for chemical components of cassava's stems, peels and leaves. Lishizhen Med Mater Med Res 22(4):908–909
Li SS, Dai HF, Zhao YX, Zuo WJ, Li XN, Mei WL (2012) Chemical constituents from the stems of cassava (Manihot Esculenta) in Hainan. J Trop Subtrop Bot 20(2):197–200
Liu H, Huang J, Liu Z, Yunze R, Yang J (2016) Antifungal effects of extracts of the cassava organ on Fusarium oxysporum f. sp. cubense in banana. J Yunnan Agric Univ (Nat Sci) 31(03):556–561
Chaparro JM, Sheflin AM, Manter DK, Vivanco JM (2012) Manipulating the soil microbiome to increase soil health and plant fertility. Biol Fertil Soils 48(5):489–499
Silva W, Deverall B, Lyon B (1998) Molecular, physiological and pathological characterization of Corynespora leaf spot fungi from rubber plantations in Sri Lanka. Plant Pathol 47(3):267–277
John J, Patil R, Joy M, Nair A (2006) Methodology of allelopathy research: I. Agroforestry systems. Allelopathy J 18(2):173
Elisante F, Tarimo MT, Ndakidemi PA (2013) Allelopathic effect of seed and leaf aqueous extracts of Datura stramonium on leaf chlorophyll content, shoot and root elongation of Cenchrus ciliaris and Neonotonia wightii. Am J Plant Sci 4:2332–2339
Ercisli S, Esitken A, Turkkal C, Orhan E (2005) The allelopathic effects of juglone and walnut leaf extracts on yield, growth, chemical and PNE compositions of strawberry cv. Fern. Plant Soil Environ 51(6):283–287
Rejila S, Vijayakumar N (2011) Allelopathic effect of Jatropha curcas on selected intercropping plants (green chilli and sesame). J Phytol 3(5):1–3
Han X, Cheng Z, Meng H, Yang X, Ahmad I (2013) Allelopathic effect of decomposed garlic (Allium sativum L.) stalk on lettuce (L. sativa var. crispa L.). Pak J Bot 45(1):225–233
Souto X, Gonzales L, Reigosa M (1994) Comparative analysis of allelopathic effects produced by four forestry species during decomposition process in their soils in Galicia (NW Spain). J Chem Ecol 20(11):3005–3015
He B, Gu M, Kang L, Lu Q (2014) A solution culture method for cassava seedling. CN 10358337 A [P], pp 2–19
Tawaraya K, Hashimoto K, Wagatsuma T (1998) Effect of root exudate fractions from P-deficient and p-sufficient onion plants on root colonisation by the arbuscular mycorrhizal fungus Gigaspora margarita. Mycorrhiza 8(2):67–70
Rinez A, Daami-Remadi M, Ladhari A, Omezzine F, Rinez I, Haouala RJAJMR (2013) Antifungal activity of Datura metel L. organic and aqueous extracts on some pathogenic and antagonistic fungi. Afr J Microbiol Res 16:1605–1612
Cheng W, Zhang Q, Coleman DC, Carroll CR, Hoffman CA (1996) Is available carbon limiting microbial respiration in the rhizosphere? Soil Biol Biochem 28(10–11):1283–1288
He K, Huang Z (1987) Rubber tree cultivation in the northern margin of tropic. Guangdong Science and Technology Press, Guangzhou
Li KY, Huang J, Lu X, Yan Q, Zhang Z, Yang B, Liu X, Huang W (2007) Regulation of cassava cultivation [S]. DB/T105-2007. Hainan Bureau of Quality and Technical Supervision, Haikou
Lu R (2000) Analysis Methods of Soil Agricultural Chemistry, vol 107. China Agricultural Science Technology Press, Beijing, pp 147–150
Guan S (1986) Soil enzymes and their research methodology. Agriculture Press, Beijing
Ch QIAN (1999) Microbiology experiment methodology. Peking University Press, Beijing
Williamson GB, Richardson D (1988) Bioassays for allelopathy: measuring treatment responses with independent controls. J Chem Ecol 14(1):181–187
Wu YH, Tian XH, Tong YA, Nan XX, Zhou M, Hou YH (2010) Assessment of integrated soil fertility index based on principal components analysis [J]. Chin J Ecol 29:173–180
Muzell Trezzi M, Vidal RA, Balbinot Junior AA, von Hertwig Bittencourt H, da Silva Souza Filho AP (2016) Allelopathy: driving mechanisms governing its activity in agriculture. J Plant Interact 11(1):53–60
Song W, Jia J, Yun X (2010) Allelopathy mechanism of fresh celery root extracts on cucumber Fusarium oxysporum: spore morphology and growth change after fresh celery root extracts treatment. J Inner Mong Agric Univ (Nat Sci Ed) 31(3):100–105
Liu S, Lv X (2014) Study on the inhibition effects of aqueous extracts from jinxiang garlic straw on Botrytis cinerea of cucumber. J Jining Univ 35(6):5–10
Qi Y (2008) Study on synergistic effect of root allelochemical and pathogen in the replant disease of strawberry (Fragaria ananassa Duch.). Hebei Agriculture University, Hebei
Liu G, Yang Q, Li L, SUN J (2008) Intercropping advantage and contribution of above and below ground interactions in wheat maize intercropping. Plant Ecol 32:477–484
Acosta-Martínez V, Zobeck T, Gill T, Kennedy A (2003) Enzyme activities and microbial community structure in semiarid agricultural soils. Biol Fertil Soils 38(4):216–227
Schippers B, Bakker AW, Bakker PA (1987) Interactions of deleterious and beneficial rhizosphere microorganisms and the effect of cropping practices. Annu Rev Phytopathol 25(1):339–358
Ilieva-Makulec K, Olejniczak I, Szanser M (2006) Response of soil micro-and mesofauna to diversity and quality of plant litter. Eur J Soil Biol 42:S244–S249
Ma H, Xu J, Zheng C, Sun X, Shu H (2011) Effects of continuous cropping system on the soil physical-chemical properties and biological properties of Gerbera jamesonii. Sci Agric Sin 44(18):3733–3740
Wang ZG, Jin X, Bao XG, Li XF, Zhao JH, Sun JH, Christie P, Li L (2014) Intercropping enhances productivity and maintains the most soil fertility properties relative to sole cropping. PLoS One 9(12):e113984
Wang Y, Wang H (2008) Research advance on allelopathy of continuous cropping plantation. World For Res 4:25–30
Larsonde RA (2013) Introduction to floriculture. Academic Press, Cambridge
Xia H, Wang L, Jiao N, Mei P, Wang Z, Lan Y, Chen L, Ding H, Yin Y, Kong W (2019) Luxury absorption of phosphorus exists in maize when intercropping with legumes or oilseed rape—covering different locations and years. Agronomy 9(6):314
Yap VY, Xaphokhame P, de Neergaard A, Bech Bruun T (2019) Barriers to agro-ecological intensification of smallholder upland farming systems in lao PDR. Agronomy 9(7):375
Xia H, Wang L, Xue Y, Kong W, Xue Y, Yu R, Xu H, Wang X, Wang J, LIU Z (2019) Impact of increasing maize densities on agronomic performances and the community stability of productivity of maize/peanut intercropping systems. Agronomy 9(3):150
Xu H, Bi H, Gao L, YUN L (2019) Alley cropping increases land use efficiency and economic profitability across the combination cultivation period. Agronomy 9(1):34
Goh Y, Marzuki N, Liew Y, GOH K (2018) Antagonistic effects of fungicolous ascomycetous Cladobotryum Semicirculare on Rigidoporus Microporus white root disease in rubber trees (Hevea brasiliensis) under in vitro and nursery experiments. J. Rubb. Res. 21(1):62–72
Acknowledgements
This work was supported by funds from the National Natural Science Foundation of China (31560374) and the Earmarked Fund for China Agriculture Research System (CARS-33).
Author information
Authors and Affiliations
Contributions
Methodology: ZL, PL, and FA; investigation: ZL, PL, and XM; writing—original draft preparation: ZL, FA, TY, and LL; writing—review and editing: FA, TY, and XM; Funding acquisition: ZL and FA.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The founding sponsors played no role in study design, data collection, data interpretation, manuscript writing, or decision to submit the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Z., Liu, P., An, F. et al. Effects of cassava allelochemicals on rubber tree pathogens, soil microorganisms, and soil fertility in a rubber tree–cassava intercropping system. J Rubber Res 23, 257–271 (2020). https://doi.org/10.1007/s42464-020-00055-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42464-020-00055-7