Abstract
A spinel structure of zinc aluminate (ZnAl2O4) particles provides a prospective material for photocatalytic applications. In this work, zinc aluminate spinel was prepared by an anion-exchange method derived from layered double hydroxides (LDHs) followed by thermal treatment. The mechanism, photoactivity, and effects of both anion substitute and temperature have been investigated. The chemical composition, morphologies, phase structures, porous structures and photocatalytic properties of samples were analyzed using thermogravimetric analysis, scanning electron microscopy, energy-dispersive X-ray spectroscopy, powder X-ray diffraction, gas sorption analysis, and ultraviolet–visible spectroscopy in detail. The results of mesoporous α-ZnAl2O4 indicate that average crystallite size, total pore volume, and BET surface area obtain 9.3 nm, 0.337 cm3/g, and 206.13 m2/g, respectively. Moreover, zinc aluminate spinel shows well phase after heating at 700 °C and LDHs structures were completely collapsed. The photocatalytic performances of α-ZnAl2O4 have been applied in p-nitrophenol reduction under visible light irradiation and required time at least 9 min. Finally, the new improved method constructs an excellent material for pretreatment of liquid pollutants containing high-level concentration at rapid treatment time.
Similar content being viewed by others
1 Introduction
Liquid pollutants discharged from cosmetic, paper printing, textile, and other industries contaminate the ecosystem and cause serious implications for animals, plants, and other organisms in aquatic life. Among phenolic compounds, p-nitrophenol (hereafter pNP) is widely applied in various industries, but pNP as a recalcitrant organic contaminant obtains high toxicity in the aquatic environment [1,2,3]. Recently, several techniques such as adsorption [4], advanced oxidation [5], biological treatment [6], coagulation and flocculation [7] have been developed to reduce phenolic compounds from watery solutions. Nevertheless, the materials for the removal of pNP obtain complicated problems, including low surface area and pore volume. Therefore, finding an inexpensive cost, the environmentally friendly method is necessary for handling industrial wastewater containing phenolic compounds.
Semiconductor photocatalysis is a prospective method to remove credential organic pollutants in wastewater. Up to now, numerous active-photocatalysts such as CuO [8], Fe2O3 [9], SnO2 [10], TiO2 [11], WO3 [12], and ZnO [13] are highly potential applications in various industries. For an effective candidate and an efficient treatment, the development of photocatalysts with high pore volume and surface area is essential. Furthermore, the previous semiconductors obtain fundamental problems such as high cost and low rates of degradation. Therefore, an excellent technique is being applied to the expandable properties of photocatalysts. This solution can be modified by compositions and morphologies of the materials. Recently, several mixed metal oxides with spinel structures as photocatalysis applications under solar light irradiation have been discussed for the treatment of organic contaminants in water [14,15,16,17].
Mixed metal oxides, also known as spinels, have been broadly reported for their great applications in the subjects of catalysis [18, 19], adsorption [20, 21], electrochemistry [22], and others. Spinels contain cationic and anionic sites with a 3:4 ratio, respectively. These materials can be represented by the general formula AB2X4, A and B refer to cations and X describes anion. Based on their anions, the spinels possess three groups such as oxyspinel (O2−), selenospinel (Se2−), and thiospinel (S2−). These groups obtain several subgroups such as spinel (A2+ B3+2 O4), ulvöspinel (A4+ B2+2 O4), bornhardtite (A2+ B3+2 Se4), tyrrellite (A1+ B3:5+2 Se4), carrollite (A1+ B3:5+2 S4), and linnaeite (A2+ B3+2 S4) [23]. Most especially, spinels offer an efficient path [24], high catalytic activity [25], improved stability [26], useful catalyst [27,28,29,30], and great recyclability [31]. The chemical structure of binary spinel (AB2O4) can be replaced by divalent (A = Zn2+) and trivalent (B = Fe3+) metal ions, resulting in photocatalytically active microstructure of zinc ferrite (ZnFe2O4) [32]. Furthermore, the atomic ratio between A2+ and A3+/A4+ acquired high catalytic activity for dye degradation [33, 34]. Nowadays, the solar-responsive spinel photocatalyst has been presented as a potential material for application in wastewater treatments [35]. Besides, oxyspinels have been studied as excellent photocatalytic degradation for methylene blue [36, 37] and methyl orange [38].
Meanwhile, several oxyspinels were prepared from inorganic anions (carbonate, nitrate, phosphate, and sulfate) pillared A2+–A3+ layered double hydroxides and followed by a thermal process that they performed the improved catalytic activities [39,40,41,42,43,44,45,46]. Afterward, we study the improved photocatalytic degradation of pNP through a solar-responsive oxyspinel derived from organic anion pillared LDH precursors under UV-light irradiation.
The aims of this work were to investigate the effects of both anion replacement and thermal treatment of starting organic anion pillared LDH precursors and to study mechanism and photoactivity of the catalyst candidate for removing an organic pollutant in wastewater.
2 Experimental section
2.1 Materials
In this experiment, aluminum nitrate nonahydrate (Al(NO3)3⋅9H2O), p-nitrophenol (pNP, > 99%), and sodium borohydride (NaBH4) were acquired from Aladdin Chemical Co. Ltd. Sodium hydroxide (NaOH), kalium hydroxide (KOH), and N,N-dimethylformamide (DMF, 99.5%) were provided by Xilong Chemical. Zinc nitrate (Zn(NO3)2⋅6H2O, 98%) and perylene 3,4,9,10-tetracarboxylic dianhydride (PTCDA, 97%) were used as supplied by Sigma-Aldrich. All chemicals were analytical grade and without further purification before use.
2.2 Synthesis of NO3ˉ (H2O) ZnAl-layered double hydroxide (LDH)
The ZnAl-NO3-LDH (hereafter ZAN-LDH) was prepared using a co-precipitation technique. Firstly, the solution containing 0.1 M Zn(NO3)2⋅6H2O and 0.05 M Al(NO3)3⋅9H2O was dissolved in CO2-free deionized water to form a blended solution. Secondly, the mixed solution was added by NaOH (2 M) until pH 7 in nitrogen ambience to avoid impurity by carbon dioxide. The third point, the resulted slurry was aged at 70 °C for 18 h. Also, the suspension was centrifuged and washed with sterile water repeatedly. Finally, the solid material was dried in the oven at 80 °C overnight.
2.3 Synthesis of perylene-3,4,9,10-tetracarboxylic acid
Perylene-3,4,9,10-tetracarboxylic acid (abbreviated as PTCA) was synthesized from hydrolyzed perylene-3,4,9,10-tetracarboxylic dianhydride (hereafter PTCDA) according to previous reported literatures [47, 48]. The PTCDA (1 mmol) was dissolved by 5% KOH solution under stirring between 60 and 70 °C. Furthermore, the solution was adjusted to acid (pH 5–6) with 0.1 M HCl after cooling to ambient temperature. In addition, the red suspension was filtered from the solution and the solid yield was directly dried in vacuum.
2.4 Synthesis of PTCA (DMF) ZnAl LDH
PTCA-intercalated LDH (denoted as ZAP-LDH) was prepared by an ion-exchange method as following: 1 gr ZAN-LDH product was dispersed in 50 mL distilled water and added to 0.5 g PTCA dissolved in 50 mL DMF. Furthermore, the suspension was adjusted at neutral condition (pH 7) using 2 M NaOH and vigorously stirred for 6 h at 70 °C. Besides, the obtained product was recovered by filtration, washed with deionized water, and dried at 80 °C overnight.
2.5 Synthesis of ZnAl2O4 spinel derived from ZAP-LDH
The calcination of solid material was constructed by heating at 700 °C and 800 °C. The dried precipitates were loaded to a ceramic boat holder, heated into a tubular furnace to the required temperatures and the heating rate was maintained at 5 °C min−1 combined with argon inflow. Each product was labeled as α-ZnAl2O4 and β-ZnAl2O4, where ZnAl2O4 describes calcined ZAP-LDH. Also, α and β represent the temperature of the thermal process at 700 °C and 800 °C, respectively.
2.6 Structural characterizations
All samples were characterized by PXRD, SEM, EDX, TGA-DTA, N2 adsorption–desorption, and UV–Vis spectroscopy. The PXRD patterns for ZAN-LDH, PTCA, ZAP-LDH, α-ZnAl2O4, and β-ZnAl2O4 were performed using a SHIMADZU LabX XRD-6000 with Cu radiation at 40 kV and 30 mA (2θ in the range 3–70° for ZAN-LDH, PTCA, ZAP-LDH, and the range from 20° to 70° for α-ZnAl2O4 and β-ZnAl2O4). The average crystal size in nm (DXRD) was calculated from XRD peaks using Scherrer’s formula (1):
where β, λ, and θ represent the full width at half maximum or FWHM of peak (radian), the wavelength of Cu Kα radiation (1.54056 Å), and the Bragg angle (°), respectively.
The surface morphology of solid products and UV–Vis absorbance spectra of the catalyst samples in the region of 250–475 nm were observed by a JSM-6510A field-emission scanning electron microscope and a SHIMADZU UV-2450, respectively. The EDX spectra of chemical composition were obtained using JEOL JED-2300 at the accelerating voltage of 20 kV. In addition, thermogravimetric analyses at a heating rate of 10 °C min−1 in nitrogen atmosphere from environmental temperature to 700 °C were recorded on a SHIMADZU DTG-60 and N2 adsorption–desorption measurements at liquid nitrogen temperature (− 196 °C) were determined using a Micromeritics instruments TriStar II 3020.
2.7 UV-light photocatalytic performance
Photocatalytic degradation of pNP was prepared by taking 0.025 mL pNP (0.6 mM and pH ~ 12), 0.75 mL NaBH4 (0.2 M), and 2.7 mL distilled water in test tubes containing 20 mg of catalyst. All tests were repeated in duplicate and a blank evaluation was also analyzed by the same procedure without a catalyst. The decolorization of pNP due to adsorption was measured from initial and final observations.
3 Results and discussion
3.1 X-ray powder diffraction analysis
Investigated materials, such as average crystallite size and phase structure from diffractograms, are shown in Fig. 1. The ZAN-LDH was synthesized by the co-precipitation technique in H2O at 70 °C. Typical properties of ZAN-LDH as a precursor were confirmed by a PXRD measurement. Furthermore, the representative signals are observed peaks at 2θ = 9.951°, 19.891°, and 29.494° corresponding to the (003), (006), and (009) planes, respectively. Consequently, the angle of nitrate ion can be determined less than 90° from the host layers.
The ZAP-LDH was prepared by an anion-exchange route from the ZAN-LDH precursor in DMF at 70 °C. New X-ray diffraction peaks of ZAP-LDH obtain at 2θ = 4.853°, 9.865°, and 14.258°, which can be indexed to (003), (006), and (009) planes, respectively. Therefore, a new basal spacing (d003 = 1.82 nm) represents evidence of new pillared material. The nitrate in the interlayer structure of ZAN-LDH is replaced by the PTCA-organic compound indicating relatively weak interaction between layered sheets and NO3ˉ anion. The illustration of PTCA intercalated LDH interlayers is presented in Scheme 1.
The ZAP-LDH has electrostatically interacted between layered sheets and carboxylate groups of PTCA as an intercalated complex. The interaction of negatively charged PTCA, positively charged layers, and water confirms hydrogen-bonding. Furthermore, the XRD pattern of ZAP-LDH presents two significant parameters such as α and c. The α parameter defines average cation-cation length within layers (2d110) and also c parameter describes Coulomb force between layer and anion in interlayer spacing (3d003). Accordingly, the c-axis value of ZAP-LDH material shows an increase. Table 1 presents the indexing of X-ray patterns between ZAN-LDH and ZAP-LDH.
Thermal evolution constructs ZAP-LDH into ZnAl2O4, a spinel structure. Besides, the specific surface area of ZnAl2O4 increases in size after calcination treatment. Furthermore, the calcined product obtains centered cubic spinel phases (JCPDS No. 05-0669) as ZnAl2O4 and diffraction peaks at 2θ = 31.4°, 36.9°, 44.8°, 49.1°, 55.7°, 59.4°, and 65.3°, corresponding to the reflections from (220), (311), (400), (331), (442), (511), and (440) crystal planes, respectively. Figure 2 shows the X-ray patterns of the spinel obtained at calcination temperatures at 700 °C (α-ZnAl2O4) and 800 °C (β-ZnAl2O4).
The standard crystal of the ZnAl2O4 spinel obtained a good index at different temperatures in which the material equals a cubic configuration and acquires a good crystallinity. Based on Scherrer's equation, the average sizes of α-ZnAl2O4 and β-ZnAl2O4 specimens calculated on the peak at 2θ = 36.75° are 9.3 nm and 10.1 nm, respectively.
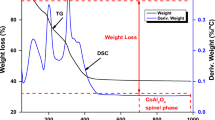
3.2 Thermogravimetric analysis and differential thermal analysis
Figure 3 illustrates the TGA-DTA curves between ZAN-LDH and ZAP-LDH under nitrogen gas. Two endothermic points at 90 °C and 230 °C prove removal from interlayer water (3.9%) and dehydroxylation (12.2%), respectively (Fig. 3a). In contrast, the endothermic peaks indicate displacement of weakly adsorbed water at 69 °C and removal of interlayer water at 181 °C. Further, nitrate ions decomposition yields 22% at a temperature between 304 °C and 582 °C. Moreover, a strong exothermic peak denotes the decomposition of PTCA (34%) at 482 °C (Fig. 3b).
3.3 Brunauer–Emmett–Teller surface area analysis
The nitrogen adsorption-desorption isotherms illustrate in Fig. 4. On the one hand, the ZAP-LDH obtains a microporous structure and follows type I sorption isotherm, indicating relatively small external surfaces and successfully intercalated by PTCA as a pillared compound. The ZAP-LDH results a significant porosity and an important surface area at least 72.96 m2/g. The type I corresponds with activated carbons, molecular sieve zeolites, and porous oxides.
On the other hand, type IV isotherms with H3 hysteresis loops are followed by α-ZnAl2O4 and β-ZnAl2O4, indicating a mesoporous scale. Calcined samples apply to rise slit-shaped pores and to improve specific surface area. The increasing temperature between 700 and 800 °C significantly reduces the specific surface area from 206.13 to 155.79 m2/g. In addition, the heating process shapes crystalline ZnAl2O4, but the carbonization removes organics as carbon cores and water from material to obtain porous particles. The surface area, pore volume, and average size of the samples are summarized in Table 2.
These burned materials construct increasing in sizes and pore volumes in a mesoporous scale at a temperature above 550 °C. Furthermore, nitrogen adsorption–desorption isotherms confirm slit-like structures on the pores as favorable porous materials.
3.4 Scanning electron microscopy morphology analysis
Scanning electron microscopy (SEM) surfaces material morphologies of ZAP-LDH, α-ZnAl2O4, and β-ZnAl2O4 are shown in Fig. 5. A uniformly ZAP-LDH sample derives numerous and regular pores as flake-like structures (Fig. 5a). The calcined samples obtain different morphologies depending on temperatures. For example, eliminated organic compounds as carbon cores and volatile substances assert lamellar structures breakdown into small pieces with pores at 700 °C and 800 °C (Fig. 5b, c). However, an anion-exchange route followed by heating treatment turns ZAP-LDH material to carbon dioxide, mixed metal oxides as spinel-type, and water. In Fig. 5d, the chemical composition from the EDX spectra reveals that the ratio of stoichiometric atom concentration is Zn:Al:O ≈ 14.6%:30.54%:54.86% ≈ 1:2:4, indicating that the as-obtained product is ZnAl2O4.
3.5 Comparison method
Table 3 offers different methods as a comparison from some physical properties. In this work, the results of ZnAl2O4 obtain a high surface area, a superior route, and comparable material. Otherwise, the other ways need a high energy source and outcome low surface area, but this anion-exchange technique followed by calcination receives an efficient energy source for synthesis. Also, the anion-substitute process acquires high pore volume, small crystallite size, and high surface area. This process produces suitable materials for catalytic purposes.
The photocatalytic reaction process of the α-ZnAl2O4 catalyst was studied by reducing pNP to pAP and was recorded using a UV–Vis spectrometer. Scheme 2 illustrates a reduction from pNP to pAP with excess sodium borohydride in aqueous solution to evaluate catalytic properties at several reaction times. The complete hydrogenation was identified by changing color from bright yellow (pNP) to colorless (pAP). Besides, the conversion of pNP to pAP was changed the nitro group by the amine group. The addition of NaBH4 as a reducing agent presents an effective technique to reduce pNP with metal catalysts under alkaline conditions. The reduction mechanism of pNP in aqueous solution over α-ZnAl2O4 can be expressed as







Sodium borohydride (NaBH4) in aqueous solution forms sodium cation (Na+) and borohydride anion (BH4ˉ). The BH4ˉ fabricates activated hydrogen (Hˉ) and metaborate (BO2ˉ) after reacting with H2O. Furthermore, the combination of activated hydrogen and α-ZnAl2O4 obtains new species as H-α-ZnAl2O4 on the surface of the catalyst. Hydrogen atom on alcohol functional group of pNP is interacted with the hydrogen of borohydride anion to fabricate p-nitrophenolate and H2. However, the oxygen on the nitro group of p-nitrophenolate is substituted by activated hydrogen during the reaction to form the amine group of pAP via electron transfer on the catalyst surface. Therefore, the resulted pAP is desorbed from the catalyst surface and the final reaction produces other metaborate anions.
The catalytic reaction can be completed within 9 min when the α-ZnAl2O4 spinel as a catalyst has been added into the cuvette. The best catalytic capacity possesses high surface area and rate of surface absorption. Adsorption of the reactant on the catalyst takes place electron transfers from BH4ˉ to pNP. First, the pNP reacts with NaBH4 to produce p-nitrophenolate (λmax = 400 nm). Second, the p-nitrophenolate and excess borohydride obtain an independent reaction in the presence of α-ZnAl2O4 spinel. In addition, a peak intensity of p-nitrophenolate presents a decrease rapidly corresponding in the color from bright yellow to colorless, but a new intensity of p-aminophenol peak (λmax = 300 nm) appears an increase gradually (Fig. 6).
4 Conclusions
This study has been investigated by visible-light-active α-ZnAl2O4 calcined from anion-exchange LDH. The effect of anion exchange LDH followed by the heat process presents the best material of the mesoporous scale. The α-ZnAl2O4 spinel provides more active sites to adsorb reactant molecules as photocatalysts. Complete conversion of p-nitrophenol to p-aminophenol was reached after 9 min under visible irradiation. Furthermore, total pore volume and BET surface area were obtained 0.337 cm3/g and 206.13 m2/g, respectively. In conclusion, the active α-ZnAl2O4 spinel is claimed as a high surface area material, a comparable product, a superior method, and an effective reducing agent.
Code availability
92E99.
References
Ren Y, Zhou J, Pan Z, Lai B, Yuan D (2019) Rapid removal of ultra-high concentration p-nitrophenol in aqueous solution by microwave enhanced Fe/Cu bimetallic particle (MW-Fe/Cu) system. Environ Technol 40(2):239–249
El-Shafey SE, Fathy NA, Khalil LB (2017) Abatement of p-nitrophenol from aqueous solutions using oxidized carbon fiber. Egypt J Chem 60(6):995–1006
You N, Li JY, Fan HT, Shen H (2019) In-situ sampling of nitrophenols in industrial wastewaters using diffusive gradients in thin films based on lignocellulose-derived activated carbons. J Adv Res 15:77–86
Cheng M, Jiang J, Wang J, Fan J (2019) Highly salt resistant polymer supported ionic liquid adsorbent for ultrahigh capacity removal of p-nitrophenol from water. ACS Sustain Chem Eng 7(9):8195–8205
Ren W, Gao J, Lei C, Xie Y, Cai Y, Ni Q, Yao J (2018) Recyclable metal-organic framework/cellulose aerogels for activating peroxymonosulfate to degrade organic pollutants. Chem Eng J 349:766–774
Elisashvili V, Kachlishvili E, Asatiani MD (2018) Efficient production of lignin-modifying enzymes and phenolics removal in submerged fermentation of olive mill by-products by white-rot basidiomycetes. Int Biodeter Biodegr 134:39–47
Yelatontsev D, Suprunchuk V, Voloshin M (2017) Sedimentation of pollutant-containing aggregates during purification of wastewater from coking plants. East-Eur J Enterp Technol 6(10):38–44
Sahu K, Singh J, Mohapatra S (2019) Catalytic reduction of 4-nitrophenol and photocatalytic degradation of organic pollutants in water by copper oxide nanosheets. Opt Mater 93:58–69
Mohan BS, Ravi K, Anjaneyulu RB, Sree GS, Basavaiah K (2019) Fe2O3/RGO nanocomposite photocatalyst: effective degradation of 4-nitrophenol. Phys B 553:190–194
Sinha T, Ahmaruzzaman Md, Adhikari PP, Bora R (2017) Green and environmentally sustainable fabrication of Ag-SnO2 nanocomposite and its multifunctional efficacy as photocatalyst and antibacterial and antioxidant agent. ACS Sustain Chem Eng 5(6):4645–4655
Mahy JG, Paez CA, Carcel C, Bied C, Tatton AS et al (2019) Porphyrin-based hybrid silica-titania as a visible-light photocatalyst. J Photochem Photobiol 373:66–76
Tong H, Zhan X, Tian X, Li J, Qian D et al (2018) Understanding the energy level matching relationships between semiconductor photocatalysts and organic pollutants for effective photocatalytic degradations. J Colloid Interface Sci 526:384–391
Nguyen VQ, Baynosa ML, Nguyen VH, Tuma D, Lee YR et al (2019) Solvent-driven morphology-controlled synthesis of highly efficient long-life ZnO/graphene nanocomposite photocatalysts for the practical degradation of organic wastewater under solar light. Appl Surf Sci 486:37–51
Benhebal H, Wolfs C, Kadi S, Tilkin RG, Allouche B et al (2019) Visible light sensitive SnO2/ZnCo2O4 material for the photocatalytic removal of organic pollutants in water. Inorganics 7(6):77–98
Asl EA, Haghighi M, Talati A (2019) Sono-solvothermal fabrication of flowerlike Bi7O9I3-MgAl2O4 p-n nano-heterostructure photocatalyst with enhanced solar-light-driven degradation of methylene blue. Sol Energy 184:426–439
Kumar A, Rout L, Achary LSK, Mohanty SK, Dash P (2017) A combustion synthesis route for magnetically separable graphene oxide-CuFe2O4-ZnO nanocomposites with enhanced solar light-mediated photocatalytic activity. New J Chem 4:10568–10583
Behera A, Kandi D, Majhi SM, Martha S, Parida K (2018) Facile synthesis of ZnFe2O4 photocatalysts for decolourization of organic dyes under solar irradiation. Beilstein J Nanotechnol 9:436–446
Xu Y, Lin Z, Zheng Y, Dacquin JP, Royer S et al (2019) Mechanism and kinetics of catalytic ozonation for elimination of organic compounds with spinel-type CuAl2O4 and its precursor. Sci Total Environ 651(2):2585–2596
De K, Mukhopadhyay C (2018) ZnFe2O4 nanoparticles: an efficient and recyclable catalyst for the synthesis of isatinylidenethiazol-4-one derivatives. ChemistrySelect 3(24):6873–6879
Appiah-Ntiamoah R, Baye AF, Gadisa BT, Abebe MW, Kim H (2019) In-situ prepared ZnO-ZnFe2O4 with 1-D nanofiber network structure: an effective adsorbent for toxic dye effluent treatment. J Hazard Mater 373:459–467
Gopinath A, Kadirvelu K (2019) Preparation and characterization of mixed metal oxide ZnCo2O4 spinel coated ACF for environmental remediation. Mater Res Express 6(4):6518–6538
Euch SEL, Bricault D, Cachet H, Sutter EMM, Tran MTT et al (2019) Temperature dependence of the electrochemical behavior of the 690 Ni-base alloy between 25 and 325 °C. Electrochim Acta 317:509–520
Bosi F, Biagioni C, Pasero M (2019) Nomenclature and classification of the spinel supergroup. Eur J Mineral 31(1):183–192
Regulska E, Breczko J, Basa A (2019) Pristine and graphene-quantum-dots-decorated spinel nickel aluminate for water remediation from dyes and toxic pollutants. Water 11(5):953–968
Liu Q, Xiong Z, Syed-Hassan SSA, Deng Z, Zhao X et al (2019) Effect of the pre-reforming by Fe/bio-char catalyst on a two-stage catalytic steam reforming of bio-oil. Fuel 239:282–289
Arandia A, Coronado I, Remiro A, Gayubo AG, Reinikainen M (2019) Aqueous-phase reforming of bio-oil aqueous fraction over nickel-based catalysts. Int J Hydrogen Energy 44(26):13157–13168
Zhou R, Zhao J, Shen N, Ma T, Su Y et al (2018) Efficient degradation of 2,4-dichlorophenol in aqueous solution by peroxymonosulfate activated with magnetic spinel FeCo2O4 nanoparticles. Chemosphere 197:670–679
Xu H, Wang D, Ma J, Zhang T, Lu X et al (2018) A superior active and stable spinel sulfide for catalytic peroxymonosulfate oxidation of bisphenol S. Appl Catal B 238:557–567
Pi L, Yang N, Han W, Xiao W, Xiong Y et al (2018) Heterogeneous activation of peroxymonocarbonate by Co-Mn oxides for the efficient degradation of chlorophenols in the presence of a naturally occurring level of bicarbonate. Chem Eng J 334:1297–1308
Tangcharoen T, Thienprasert J, Kongmark C (2018) Optical properties and versatile photocatalytic degradation ability of MAl2O4 (M = Ni, Cu, Zn) aluminate spinel nanoparticles. J Mater Sci 29(11):8995–9006
Hu L, Zhang G, Liu M, Wang Q, Wang P (2018) Optimization of the catalytic activity of a ZnCo2O4 catalyst in peroxymonosulfate activation for bisphenol A removal using response surface methodology. Chemosphere 212:152–161
Yu Z, Moussa H, Chouchene B, Liu M (2019) One-step synthesis and deposition of ZnFe2O4 related composite films via SPSS route for photodegradation application. Nanotechnology 30(4):5707–5743
Kadhim SH (2018) Synthesis, characterization and catalytic activity of NiO-Mn2O3/ZrO2 spinel Co-catalysts. Asian J Chem 30(7):1650–1654
Shi Y, Tang X, Yi H, Gao F, Zhao S et al (2019) Controlled synthesis of spinel-type mesoporous Mn-Co rods for SCR of NOx with NH3 at low temperature. Ind Eng Chem Res 58(9):3606–3617
Xu X, Xiao L, Jia Y, Hong Y, Ma J et al (2018) Strong visible light photocatalytic activity of magnetically recyclable sol-gel-synthesized ZnFe2O4 for rhodamine B degradation. J Electron Mater 47:536–541
Vinosha PA, Xavier B, Anceila D, Das SJ (2018) Nanocrystalline ferrite (MFe2O4, M=Ni, Cu, Mn, and Sr) photocatalysts synthesized by homogeneous Co-precipitation technique. Optik 157:441–448
Yakob M, Umar H, Wahyuningsih P, Putra RA (2019) Characterization of microstructural and optical CoFe2O4/SiO2 ferrite nanocomposite for photodegradation of methylene blue. AIMS Mater Sci 6(1):45–51
Douafer S, Lahmar H, Benamira M, Rekhila G, Trari M (2018) Physical and photoelectrochemical properties of the spinel LiMn2O4 and its application in photocatalysis. J Phys Chem Solids 118:62–67
Chen M, Wu P, Wei Q, Zhu Y, Yang S et al (2018) The role of oxygen vacancy over ZnCr-layered double oxide in enhancing solar light-driven photocatalytic degradation of bisphenol A. Environ Chem 15(4):226–235
Huang MX, Wu X, Yi XD, Han GB, Xia WS et al (2017) Highly dispersed CoOx in layered double oxides for oxidative dehydrogenation of propane: guest-host interactions. RSC Adv 7:14846–14856
Ortega KF, Rein D, Lüttmann C, Heese J, Ӧzcan F et al (2016) Ammonia decomposition and synthesis over multinary magnesioferrites: promotional effect of Ga on Fe catalysts for the decomposition reaction. ChemCatChem 9(4):659–671
Li D, Xu S, Cai Y, Chen C, Zhan Y et al (2017) Characterization and catalytic performance of Cu/ZnO/Al2O3 water-gas shift catalysts derived from Cu-Zn-Al layered double hydroxides. Ind Eng Chem Res 56(12):3175–3183
Li D, Fan Y, Ding Y, Wei X, Xiao Y (2017) Preparation of cobalt-copper-aluminum spinel mixed oxides from layered double hydroxides for total oxidation of benzene. Catal Commun 88:60–63
Răciulete M, Layrac G, Papa F, Negrilă C, Tichit D et al (2018) Influence of Mn content on the catalytic properties of Cu-(Mn)-Zn-Mg-Al mixed oxides derived from LDH precursors in the total oxidation of methane. Catal Today 306:276–286
Huo L, Liu B, Li H, Cao B, Hu XC et al (2019) Component synergy and armor protection induced superior catalytic activity and stability of ultrathin Co-Fe spinel nanosheets confined in mesoporous silica shells for ammonia decomposition reaction. Appl Catal B 253:121–130
Meng W, Li F, Evans DG, Duan X (2004) Photocatalytic activity of highly porous zinc ferrite prepared from a zinc-iron(III)-sulfate layered double hydroxide precursor. J Porous Mat 11(2):97–105
Liu Y, Wang H, Xiong C, Chai Y, Yuan R (2017) An ultrasensitive electrochemiluminescence immunosensor for NT-proBNP based on self-catalyzed luminescence emitter coupled with PdCu@carbon nanohorn hybrid. Biosens Bioelectron 15(87):779–785
Rana U, Chakrabarti K, Malik S (2011) Insitu preparation of fluorescent polyanilinenanotubes doped with perylenetetracarboxylic acids. J Mater Chem 21:11098–11100
Stringhini FM, Foletto EL, Sallet D, Bertuol DA, Filho OC et al (2014) Synthesis of porous zinc aluminate spinel (ZnAl2O4) by metal-chitosan complexation method. J Alloy Compd 588:305–309
Anchieta CG, Sallet D, Foletto EL, da Silva SS, Filho OC et al (2014) Synthesis of ternary zinc spinel oxides and their application in the photodegradation of organic pollutant. Ceram Int 40(3):4173–4178
Gholami T, Niasari MS, Sabet M (2018) Novel green synthesis of ZnAl2O4 and ZnAl2O4/graphene nanocomposite and comparison of electrochemical hydrogen storage and Coulombic efficiency. J Clean Prod 178:14–21
Staszak W, Zawadzki M, Okal J (2010) Solvothermal synthesis and characterization of nanosized zinc aluminate spinel used in iso-butane combustion. J Alloy Compd 492(1–2):500–507
Battiston S, Rigo C, Severo EC, Mazutti MA, Kuhn RC et al (2014) Synthesis of zinc aluminate (ZnAl2O4) spinel and its application as photocatalyst. Mat Res 17(3):734–738
Saikia P, Goswamee R (2018) The effect of strength of bases and temperature on the synthesis of Zn-Al layered double hydroxides by a non-aqueous ‘soft chemical’ sol-gel method and formation of high surface area mesoporous ZnAl2O4 spinel. ChemistrySelect 3(26):7619–7626
Acknowledgements
The author thanks the State Key Laboratory of Inorganic Synthesis & Preparative Chemistry, Department of Chemistry, Jilin University-China, for material characterizations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of the author, the corresponding author states that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Putra, A.T.S.P. An improved method for high photocatalytic performance of ZnAl2O4 spinel derived from layered double hydroxide precursor. SN Appl. Sci. 2, 842 (2020). https://doi.org/10.1007/s42452-020-2682-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2682-7