Abstract
Health hazards associated with bio-aerosol is gaining immense importance in recent time. However, studies linking the probable source characterization of bio-aerosol, and their potential effect as respiratory ailments on the local people are extremely rare over the Indian subcontinent as well as in global perspective. To fill our knowledge gap, we have conducted a 3-year study on fungal bio-aerosol characterization, possible source segregation, identification of health hazardous fungal species and their role in causing allergy from seven different micro-environments over a semi-rural site of lower Indo-Gangetic Plain, West Bengal, India. The result showed the highest fungal spore concentration as well as spore diversity in the crop field (4477 ± 1343 spores m−3) and the lowest in the classroom (1994 ± 651 spores m−3). Ascospore, basidiospore, Cladosporium, and Aspergilli group were the primary indoor spores. The marker species for the crop field were Curvularia and Drechslera, whereas Ganoderma was for the factory environment. Source profile of the fungal spore of different micro-environments indicates the domination of outdoor species, whereas enclosed micro-environments have been dominated with spores of indoor origin. Aspergillus, Curvularia, Penicillium, and Rhizopus oryzae showed maximum correlation with the local allergic rhinitis and asthmatic patients. In skin prick test, Aspergillus sp., contributing a maximum percentage of the total culturable fungi, was found to be an expected potent allergic candidate in both non-asthmatic and asthmatic patients, as well. This study will directly help the inhabitants to avoid the hazardous fungal sensitization in different micro-environments.
Similar content being viewed by others
1 Introduction
Bio-aerosol, often known as airborne particles and their derivatives such as allergens, endotoxin, etc. of different biological origin, is of the major cause of respiratory ailments. Among these bio-aerosols, fungal spores represent one of the most prevalent components [36]. They can originate from both natural (soil, vegetation, debris, etc.) and human-made (building materials, organic waste, etc.) sources. Through the process of aerosolization, they disperse into their surrounding environments from the sources [35]. Being respirable by size (2–10 µm), fungal spores can easily enter the human respiratory tract [67]. Fungus-induced allergic respiratory symptoms can occur from the indoor, occupational environment or both [3, 9]. Throughout the world, 20–30% of the allergic problems are attributable to fungal spores, which include 44% atopic and 80% among asthmatics [60]. In India, about 20–30% of the population suffer from allergic rhinitis, and 15% of them suffer from asthma alone [61].
Previous studies have shown that outdoor spore concentration is dominant over the indoor [20]. Outdoor spore level is mostly governed by different meteorological factors (temperature, relative humidity, wind speed, and rainfall), the host–fungus relationship, and source strength potential [19, 27, 33]. On the other hand, indoor spore diversity is a mixture of both indoor and outdoor species [34, 39]. Growth of indoor fungi primarily depends upon the indoor moisture content, temperature, and presence of suitable growing surfaces that may cause fluctuations in fungal level [44, 47, 68]. In rural areas of eastern India, Alternaria, Aspergillus niger, Aspergillus flavus, Cladosporium cladosporioides, are reported to be the major indoor culturable fungi while ascospores, basidiospores, Alternaria, Nigrospora, and Aspergilli group are predominantly outdoor fungi [5, 6].
Numerous studies have been carried out to identify the fungal spores, which could be potential sources of allergen released in the environment. So far, around 180 fungal allergens have been identified to induce IgE-mediated hypersensitivity in atopic patients [48, 60]. A study conducted in a rural area with tropical climate from eastern India has revealed that 95.1% of atopic patients were sensitized to A. fumigatus through skin prick test (SPT) [6]. Both the young children and the respiratory allergic patients have been reported to display potential health issues on fungal spores’ exposure [16, 63]. However, very little is known about the spore concentration, seasonal distribution, and their relation with allergic sensitization among the local people in rural and semi-rural regions of eastern India. These regions have higher vegetation coverage and can contribute to a higher spore source than urban areas. Therefore, it is essential to conduct a study on both spore concentration and their probable sources over different environments of rural or semi-rural areas and then to determine their potential impact on the health of local people in terms of allergic sensitization. This could improve awareness among the local people by forecasting through an allergist for the up-gradation of health outcomes of the sensitized patients.
The present study aims to investigate the potential sources and spatiotemporal variation of fungal spores over different environments within the geographical location of Habra, a semi-rural site in eastern India. Further studies are done to check the seasonal variation of culturable fungi in different micro-environments and their role in causing allergic disease in people residing in those areas. The study also focuses on determining the probable allergenic impact of exposed dominant aero-mycoflora from different environments through extract-based SPT.
2 Materials and methods
2.1 Study design, location, and characteristics of sampling sites
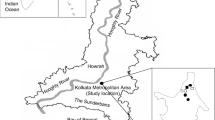
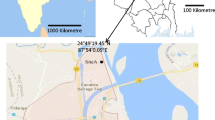
This aero-mycological study was carried out in a semi-rural site (Habra, latitude: 22°49′48.00″ N and longitude: 88°37′48.00″ E) of lower Indo-Gangetic Plain (IGP), West Bengal, the eastern part of India (Fig. 1). Total seven different environments such as bank, hospital, factory, crop field, classroom, library, and institutional office room were selected in Habra. Each of these environments is considered as micro-environments, and their characteristics are described in Online Appendix A, Table. A.1. The selected area is surrounded by the lush greenery of tropical moist deciduous forest, horse-shoe shaped lake and agricultural field. According to provisional reports of Census India, 2011, the population of Habra was 147,221. The principal occupation of the people is agriculture, and paddy is the primary cultivar here. However, very recently, due to the construction of many housing projects and investment activities for commercial as well as industrial purposes, the area became populated. Thus, the study site perfectly executes the features of a semi-rural area over the eastern part of India.
2.2 Sampling, instrumentation and sample analysis
The total fungal spore as well as culturable fungal spore content of the air was measured in all the seven micro-environments of Habra from May 2014 to April 2017, using the Burkard Personal Slide sampler (Burkard Manufacturing Co.Ltd. Hertfordshire WD3 1PJ, UK) and Andersen Two-Stage sampler ([10]; manufactured by Thermo Andersen, Smyrna, 300082-5211, USA), respectively. The sampling was conducted at the height of 1.5 m above the floor or ground level. Samples were collected once in a month, in between 10.00 h and 15:30 h. After mounting of the fungal spores, trapped by Burkard Personal Slide sampler (10 ± 0.1 L min−1), the scanning was carried out under 400 × and 1000 × magnification of a Nikon high-resolution light microscope (Labophot 2, Nikon Corp., Japan). Identification of fungal spores based on morphology was made up to the genus level using the guidelines of the British Aerobiological Federation (The British Aerobiology Federation 1995) and from the previous studies [18]. Finally, the spore concentration was expressed as the number of spores per meter cube of air (spores m−3, Online Appendix B). Within total aero-spora count, we have listed a group, named “Aspergilli” (“Asp/Pen”). The conidia of this group are indistinguishable, and they are identified only with the viable method [55].
Andersen two-stage sampler with total 200 holes, having upper and lower stages of 1.5 mm and 0.4 mm of holes’ diameter, drew air (28.3 ± 0.3 L min−1) through the orifice at its top. This instrument has a particle diameter cutoff size (d50) of 8.0 µm and 0.95 µm for the upper and lower stages, respectively. Above this aerodynamic diameter, the sampler meets the cutoff size requirements for most of the fungal spores. Collection of the fungal sample was performed onto two petri-plates containing potato dextrose agar media with chloramphenicol (25 mg mL−1) added to cease the bacterial growth. Plates were then incubated at 27 ± 0.5 °C temperature for 2–7 days to get the optimum growth of fungal colonies as previously described by Sharma et al. [59]. For the identification, each and every fungal colony from the exposed petri-plate was isolated and subcultured for getting pure culture on PDA slant. Finally, the microscopic morphological identification was confirmed from Agharkar Research Institute, Pune. However, culturable airborne fungal spore counts were represented as colony-forming units per meter cube of air (CFU m−3, Online Appendix B). The growth of these spores was measured by dividing the concentration of culturable fungus with the total fungal spores’ concentration, followed by the multiplication with a hundred.
The entire sampling period was represented into four major seasons, including—summer (March–May), monsoon (June–September), post-monsoon (October–November) and winter (December–February).
2.3 Study population, hospitalization data, and questionnaire survey
We focused on pooling the patients’ data, suffering from allergic rhinitis (including sneezing, runny nose, stuffy nose, coughing, and itchy eyes), and asthma with their age, sex, admission date and professions. In 3 years, a total of 179 children and 2,156 adults, age range between 6 and 60 years, visited Habra State General hospitals, Habra, with these symptoms. Children below the age of 6 years are difficult for the diagnosis of asthma [14]. The questionnaire was also done simultaneously with the selected patients. The format of questionnaire form (Online Appendix C) was prepared from Bose Institute, Kolkata, with a slight modification of the layout used by Singh et al. [62]. It was finally translated into the local language (Bengali).
2.4 Allergenic sensitivity of atopic patients against dominant culturable aero-mycoflora through SPT
Skin prick test (SPT) was performed according to the ethics of the hospital with our supplied fungal allergen extract, along with other standard allergens. The antigenic crude extract (1:10 w/v) was prepared from the individual dominant culturable fungi of the study area [4, 65]. Phosphate buffer saline (PBS-0.01M, pH 7.2) and histamine diphosphate (1 mg mL−1) were taken as negative and positive controls, respectively. This test was performed on two hundred and sixty patients (n = 260) with prior permission from them. For individual atopic patients, 20 µL of each crude antigenic fungal extract was taken and pricked with a sterile lancet on the ventral side of the arms. After 20 min of pricking, the wheal response was measured based on gradation (1 +, 2 +, 3 +) [16].
2.5 Data analysis
Principal component analysis (PCA) using R-programming language (Version-3.4.4) was used to evaluate the possible sources of significant fungal spores over different micro-environments during the entire study period [52, 53, 69]. It is a mathematical tool that can combine information by correlating a large number of variables and transform them into a smaller set of statistically independent new variables retaining most of the data. The PCA-generated new components are linearly independent and can be interpreted physically by applying Kaiser’s varimax rotation, which can aid the physical interpretation of the principal components. Components are classified according to the eigenvalue > 1. The PCA only indicates the relative influence of each identified source to each fungal species. Several studies have been used this receptor model for the source segregation of the fungal spores [23, 24]. In this study, the factors information from PCA, along with the correlation of total fungal spore with meteorological variables, was taken for the source apportionment. Moreover, the information of marker species with a single source of origin was incorporated.
3 Results and discussion
3.1 Inter-comparison of total fungal spore count in different micro-environments
Total fungal spore concentrations in different micro-environments over a semi-rural atmosphere are shown in Fig. 2a. The highest number of fungal spore concentration was observed in the crop field (average concentration 4477 ± 1343 spores m−3), followed by the factory (average concentration 3891 ± 558 spores m−3) environment. In the classroom, fungal spore concentration was found to be lowest with an average concentration of 1994 ± 651 spores m−3. Higher outdoor spore concentration can be explained by the presence of vast vegetation and farmland in and around the sampling site which might act as a potential source for fungal spores [19].
The open field was marked by the highest fungal diversity (based on Shannon diversity index), whereas hospital showed the lowest spore diversity among the selected micro-environments. These spores diversity often depends on the sampling conditions and meteorological factors too [6, 33]. Other than unidentified ascospore and basidiospore, Cladosporium and Asp/Pen, consisting of 29–43% of the total spore load, were identified as the primary dominant species, in five indoor air (classroom, institutional office room, bank, library, and hospital). In the factory, Ganoderma and Chaetomium were found to be the dominant spore species, accounting for ~ 26% of total spore concentration. Curvularia and Drechslera were the two predominant fungal spores over the crop field, consisting of 32% of the total fungal spores. Unidentified ascospore and basidiospore significantly contribute 18–44% to the total fungal spore level for each micro-environment. This result coincides with the previous study from eastern India [16, 21].
3.2 Seasonal variation of total fungal spores
Seasonality on the total fungal spore disclosed the highest and lowest spore levels during the post-monsoon and winter season in sampling area (Fig. 3a). A significantly higher spore concentration (performed one-way ANOVA test, fvalue < fcritical value) during monsoon indicates that the fungal growth during this season exceeds the below-cloud scavenging of the fungal spore by precipitation.
The seasonal variation of Cladosporium, one of the indoor dominating species, showed the highest spore load in post-monsoon and the lowest during monsoon and winter seasons (Fig. 4). Cladosporium requires a combination of optimum meteorological factors for their growth [57] and release [56]. Low relative humidity (~ 60%), moderate wind speed (4–6 ms−1), and occurrence of precipitation events during the post-monsoon season significantly increased the concentration over the sampling station. The other indoor dominating fungal group, Asp/Pen exhibited similar seasonal variation. The release mechanism of Asp/Pen spore can be both active and passive [42]. Elevated concentration of Asp/Pen in post-monsoon was observed along with higher temperature (30 °C) and lower relative humidity (58%). This indicates the domination of active spore release mechanism of Asp/Pen during sampling time. Curvularia was identified as the marker fungi in crop field with a very high frequency during summer and post-monsoon time as well. This genus is reported to be a potent plant pathogen of plants belonging to Poaceae family and is also reported in the rice field of eastern India [17, 41]. An increase in this spore count during the harvesting months of May and November is evident from the monthly fungal calendar (Online Appendix D, Fig. D.1–7). Within the factory aero-mycoflora, Ganoderma, the dominating fungus displayed higher spore consistency during post-monsoon and lower during winter season. Large number of rainy days (~ 22 rainy days in October–November) and availability of growing material, such as wood, elevate its concentration during the post-monsoon season [8].
3.3 Source segregation of airborne fungi
PCA has been used to determine the potential source profile of airborne fungus. The eigenvalues of each component for each micro-environment have been provided in Online Appendix A, Table. A.2. We have represented two principal components (PC1 and PC2) in Fig. 5 for all micro-environments.
3.4 Sources of fungal spore in bank micro-environment
Four major potential sources can be identified from the bank aero-spora through PCA analysis. Factor 1 consists of fungi such as rust spore, Periconia, and Pithomyces. Rust spore, found mostly in outdoor, was used as a marker fungus for this factor [37]. As these fungi showed a negative correlation with wind speed (Online Appendix A, Table. A.3, r = − 0.06 to − 0.18, p value < 0.01), it could be assumed that these fungi were transported from outdoor to the indoor environment via human movement. For Factor 2, low and moderate correlation of the fungal spores, such as unidentified basidiospore, unidentified ascospore, and Curvularia with wind speed displays wind-borne as well as human-mediated transport inside the bank. Curvularia spores, used as a marker fungi for Factor 2, are majorly found in soil [7, 41]. The probable source for Cladosporium, Asp/Pen, and Torula, together correlated well with Factor 3, could be paper dust, and building material, too. From Factor 4, wood and /carpet materials would be the potential source for Nigrospora.
3.5 Sources of fungal spore in hospital micro-environment
From hospital micro-environment, three major possible sources of fungal spore have been identified. Factor 1 consists of Asp/Pen and could be interpreted as an indoor source. Previous studies have shown that wastes, such as fluid, cotton, puss, and tissues, are the good sources of Asp/Pen in hospital [51]. The positive correlation of relative humidity with Asp/Pen indicates higher growth of these fungal spores in a moist environment. The damp wall inside the hospital could be the probable source for the fungi (Cladosporium, Basidiospore, Nigrospora) of Factor 2. Fungal spores in Factor 2 also revealed a significant positive correlation with relative humidity (r = 0.3–0.59, p value < 0.1). Factor 3 (Periconia, ascospore) can be attributed to those spores which were transported inside through human activity.
3.6 Sources of fungal spore in factory micro-environment
Factor 1 from factory micro-environment is majorly loaded with Ganoderma, Curvularia, Cladosporium, and Torula. Wood is considered as a major probable source of these fungi. Spores of this factor were well correlated with relative humidity inside the factory [46]. Damp wall of the building can be interpreted as the potential sources of fungi (Periconia, Chaetomium, Nigrospora, and Pithomyces) from Factor 2. In Factor 3, positive correlation of the fungi with wind speed discloses wind-mediated (r = 0.026–0.12, p value < 0.1) transport from the outside.
3.7 Sources of fungal spore in crop field micro-environment
In the case of crop field, Cladosporium, Alternaria, and Torula in Factor 1 majorly arise due to post-harvesting activities. Dead leaf and plant debris during the post-harvesting period are considered to be a major source of these fungi [35, 53]. A negative correlation of wind speed (r = − 0.13 to − 0.2, p value < 0.1) with the fungus of Factor 1 reveals their dispersal from the nearby sources. Factor 2 could be explained by fungal spores, present during the rice-harvesting period. Curvularia and Drechslera, potential plant pathogens of Poaceae family, release in large number during the paddy harvesting time [38, 41]. Factor 3 entails fungi with mixed sources [35]. Factor 4 consists of rust spore, an outdoor fungus which infects plants of the Brassicaceae family during the winter season [37, 66].
3.8 Sources of fungal spore in classroom micro-environment
Within classroom aero-spora, the factors are not very well separated due to a different mixture of sources. Factor 1 (Torula, Chaetomium, and Coprinus) could be interpreted as wind-borne outdoor spores as they showed a significant positive correlation with wind speed (r = 0.06 to 0.31, p value < 0.1). Factor 2 (rust spore, ascospore, Asp/Pen) could be explained as mixed transport due to showing low/moderate correlation with wind speed (r = 0.01 to 0.13, p value < 0.1). Fungal species, like unidentified basidiospore, Pithomyces, Curvularia, and Cladosporium from Factor 3, were negatively correlated with wind speed. Therefore, the transport of these fungi is suggested to be a human-mediated inside the classroom. Factor 4 is characterized by Periconia, Nigrospora, and Alternaria spores, and they can be identified as indoor fungus.
3.9 Sources of fungal spore in library micro-environment
In library air, Factor 1 is loaded with Torula and Alternaria, and their probable source could be interpreted as wooden materials, and damp wall of the library. The fungus from factor 2 exhibits human-mediated transport from the outdoor air. Factor 3 representing Asp/Pen, Cladosporium and Basidiospore could be explained as cellulose degrading fungus. Previous studies in similar micro-environment indicate these fungus feed on paper, glue, and other cellulosic materials found inside a book [64].
3.10 Sources of fungal spore in institutional office room micro-environment
The PCA result of the institutional office room indicates a mixed source profile for fungal aero-spora. Factor 1 (Torula, Pithomyces, Asp/Pen, Curvularia) is principally contributed by indoor sources. These fungal spores showed a high positive correlation with both temperature and relative humidity (r = 0.26 to 0.53, and r = 0.11 to 0.44, p value < 0.1). Factor 2 and factor 3 can be identified as fungal spores with an outdoor origin. Some fungal spores in factor 2 and 3 exhibited a positive correlation (r = 0.25, p value < 0.1) with wind speed, whereas other spores displayed a negative correlation (r = − 0.01 to − 0.5, p value < 0.1).
The air inside the classroom, institutional office room, and bank micro-environments have shown the predominance of outdoor spores, contributing 85%, 75% and 65% of outside sources. Shoes, shirts, and human body help in transporting the spores indoor [39]. Moreover, transportation of fungal spore by the wind is found to be another reason to increase the indoor spore level [1]. On the contrary, the indoor ambiance of hospital and library promotes the growth of the fungi, residing inside the room [2, 68]. In such cases, indoor temperature and relative humidity were two significant factors playing a role in the growth of indoor fungi under suitable conditions. Within the factory, the same indoor and outdoor ambiance might be the result for an equal percentage of outdoor and indoor spores' source, correlated with total variance of PC. For a crop field, the cutting of trees releases a high amount of spores in the neighboring atmosphere and the spore peak is totally independent on meteorological factors [25].
3.11 Comparative analysis on the growth and seasonality of culturable fungi
The Andersen two-stage sampler measured comparatively lower number of culturable fungal spores (1366–2073 CFU m−3) than the Burkard Personal sampler (1994–4477 spores m−3). The Andersen sampler collects only those spores, which can show their growth under certain conditions. This is consistent with the previous study from eastern India [6]. From our sampling, a total of ten fungal genera (Aspergillus, Alternaria, Curvularia, Cladosporium, Penicillium, Pithomyces, Chaetomium, Candida, Rhizopus and Fusarium) and twenty fungal species were identified. Considering all the micro-environments, the comparative study on the average total concentration of culturable fungi is shown in Fig. 2b. Like total fungal spore level, culturable fungal spore count was highest in the crop field (avg. conc. 2073 ± 654 CFU m−3) and lowest in institutional office room (avg. conc. 1366 ± 377 CFU m−3). The seasonal variation in growth of culturable fungi is presented in Fig. 3b. The overall median value of growth was observed to be maximum for the classroom (69.9%), followed by library (58.8%) and institutional office room (60.7%). Presence of nearby sources of fungi along with adequate indoor temperature and moisture (Online Appendix A, Table A.4) favors the growth of culturable fungi over these three micro-environments [44]. The lowest median for growth rate was found in hospital (42.8%) followed by an outdoor crop field (43.1%) during the sampling period. Previous studies have reported low growth rate in outdoor sites due to harsh environmental condition [35].
Season-wise analysis reveals that the substantial seasonal variation with elevated culturable fungal growth was found during summer and monsoon season in the classroom and institutional office room aero-mycospora. For both of these two environments, the median percentage of growth was near 83–90%. For Aspergillus and Penicillium, the peak of growth rate was found highest during monsoon, followed by summer. During summer, low relative humidity leads to a higher spore buoyancy and facilitates the transport of spores from outdoors [22, 40]. But during monsoon season, heavy precipitation worsens the water leaking condition, which in turn increases the wall dampness and creates a perfect situation to elevate their growth [32]. In hospital and library, a seasonal variation on the growth level of culturable fungi was not prominent due to the high contribution of indoor sources (~ 60–70%). It is reported that in indoor, occupants frequently release fungi from their hair, skin, and fingernails. These are the most important source of indoor airborne fungi [68]. Also, the poor ventilation and homogenous environment over these two sites help to keep a continual growth. Similarly, the presence of a continuous indoor source of fungi, dynamics of year-long human movement and suitable growth conditions induces less variability within bank micro-environment [15, 39]. In the crop field, harvesting month of paddy plant had a direct effect on the release of Curvularia spores, and it directly increases the growth of this fungal genera. Aspergillus, the second most prevalent culturable fungi in the crop field air, registered a very low level of growth, and it comes from a mixed source. Due to unceasing fungal source, no seasonal fluctuation was noticed for Aspergillus. In the factory aero-spora, a sudden drop in growth of culturable fungi was seen during winter periods. Low relative humidity (50.6 ± 7.8%) and drought periods may hinder the development of many fungi during this season. Rhizopus oryzae, being a most prevailing culturable fungus of the factory, gained the maximum concentration in the post-monsoon season. During this time, both high temperature and moderate relative humidity facilitates the release of spore from the sources as well as the mixing of spores to the air. Within the same micro-environment, the second most dominating contributor was Aspergillus sp. This species was at its peak during the summer when high temperature and dry air facilitates their transport from the sources.
3.12 Relationship between culturable fungi with patients’ data
To assess the intensiveness of the exposure of bio-aerosol, 2335 study subjects were divided into three categories: students—spending 6–7 h of a day in the classrooms; factory workers—staying in their work an average of 8–10 h day−1, and farmers—usually spending 10–12 h in the open crop field. Lastly, regression analysis was performed with the major culturable fungal spore concentration of the corresponding micro-environments (classroom, crop field, and factory). The exacerbations of fungal allergy are correlated well with the concentrations of culturable allergic fungi rather than the total fungal concentration [5, 6, 16, 45].
During the summer season, the hospitalization records indicate the maximum number of allergic rhinitis patients exhibited a moderate to strong (strong: r ≥ 0.8; moderate: r ≥ 0.6–0.79; low: r < 0.6; [54] positive correlation with A. fumigatus (r = 0.71, p value < 0.05), A. terreus (r = 0.79, p value < 0.05) and P. oxalicum (r = 0.83, p value < 0.05) (Fig. 6a). PCA analysis reveals an outside source for Asp/Pen in the factory micro-environment. On the other hand, the maximum number of asthmatic workers visited hospital mostly during winter and monsoon periods. Several studies have reported significant associations between asthma hospitalization and colder seasons as respiratory viruses may reinforce the potential health effect during winter seasons [14]. However, very intense exposure to respirable fungal allergens may be associated with asthma in occupational environments [11, 43]. R. oryzae was found to be correlated low to moderate (r = 0.36, 0.68; p value < 0.05) with the factory workers’ asthmatic data for both winter and monsoon seasons.
Among the farmers, allergic rhinitis was found to be more common than asthma. The frequency of hospital visits and patients' admission was maximum during summer and monsoon seasons for farmers. In these two seasons, farmers' hospital admission were found to be positively related (low to average) to A. fumigatus (r = 0.52, 0.28; p value < 0.05) (Fig. 6b). Insignificant correlation with Curvularia lunata and hospitalization records indicates that this major species of cropland does not contribute too much in respiratory problem among the farmers. During the twentieth century, studies on farmers have shown that agricultural exposures may result in higher rates of chronic pulmonary diseases [58].
In classroom micro-environment, a significant correlation was observed for A. fumigatus between asthmatic and allergic rhinitis patients during summer. During monsoon and winter, A. niger, A. terreus, and Curvularia showed a strong to a medium positive correlation with asthma and allergic rhinitis (r = − 0.36 to 0.92, and r = − 0.85 to 0.61; p value < 0.05) (Fig. 6c). The occurrence of these two diseases was observed to be much high during monsoon and winter, compared to other seasons. The origin of these fungi, as concluded from PCA, is majorly outdoor, and the high growth of these fungi within the classroom might help to build up disease-causing environment.
There are some limitations of the study as our findings are mainly confined on the fungal spore, while aeroallergens also include pollen grains. But fungal spores have a higher and consistent allergenicity effect than pollen grains over the general population. It is mainly because of two reasons: comparatively higher fungal spore concentration and being easily respirable spore size than pollen [26, 61]. Though many reports have shown positive associations of fungal concentration with multiple allergic and respiratory effects [6, 11, 13, 16], some studies do not support quantitative relationships between fungal exposure and allergy [12, 50].
3.13 Allergenicity assessment of dominant culturable fungus
A precise diagnosis of the fungal sensitization is essential in both atopic and asthmatic patients. Dominant culturable fungal species (Online Appendix A, Table A.5) were selected for the allergenicity assessment through SPT upon asthmatic as well as non-asthmatic patients, suspected to have a fungal allergy. Germination of spores might be the main cause to increase allergen release from some allergic fungi [31, 65]. Hence, the exposure to allergens might be the opportunity for spores to germinate after inhalation.
In the selected sampling area, 60% of a total of asthmatic patients (n = 86) and 49% of non-asthmatic patients (n = 174) were found to have sensitivity against one of those tested fungal species. Figure 6d represents that the percentage of atopicity to individual fungal species was higher among the asthmatic patients as compared to the non-asthmatic ones. The extract of A. fumigatus had shown the strongest fungal sensitization, with 48% in asthmatics and 35% in non-asthmatic patients. The Asian population with severe asthma has noted maximum allergenic reactivity in Aspergillus sp. through SPT [29, 30]. The highest degree of sensitization (3 +) was also found for A. fumigatus (Online Appendix A, Table A.6). A low percentage of sensitivity was noticed to the extract of C. lunata (7.8% in asthmatics) and C. cladosporioides (14.3% in non-asthmatics). Around Ambala city of Haryana, northern India, A. flavus, A. fumigatus, and Penicillium sp. are reported to be as the major allergens through SPT among the patients with asthma, and allergic rhinitis [49]. But our findings unearthed Penicillium sp. to be as a most sensitive fungus in non-asthmatic patients. In previous studies, the risk of hypersensitivity to Penicillium is reported among asthmatic children than among adults [28].
Therefore, in this present study, the potential adverse health effect due to mold exposure and mold allergenicity was determined for asthmatic and non-asthmatic patients too. Aspergillus species, contributing up to 60% of total culturable fungi, were found to be the most likely active causative candidate for atopic as well as asthmatic patients.
4 Conclusions
In a semi-rural area of eastern India, studies to identify the possible sources of fungi, their overall contribution pattern in relation to meteorological parameters, patients-specific exposure and sensitization profile have never been attempted before. From this study, we can conclude that (1) fungal spores can have different potential sources; however, maximum vegetation coverage was the main reason for flourishing the highest spore concentration in a semi-rural area, especially for outdoor environments. On the contrary, the indoor environment had the mixture of possible spore sources, (2) the seasonal fluctuation on the total spore concentration was noticeable. In all the sampling micro-environments, maximum spore load during post-monsoon season might be due to the effect of meteorological factors, and crop harvesting. (3) In indoor, maximum median growth level for culturable fungi was due to the presence of suitable growth conditions. In this case, the seasonality did not affect them. (4) Aspergillus sp. and Penicillium sp. showed maximum correlation with allergic rhinitis and asthmatic patients among the factory workers, farmers, and students, and (5) Culturable fungi could be associated with allergic reactions among both asthmatic and non-asthmatic patients, respectively. Aspergillus species, contributing a maximum percentage of the total culturable fungi, were found to be the most likely potential allergenic candidate in both asthmatic and non-asthmatic patients. However, this study gives useful insight for the local people to plan their daily activities for lowering the aeroallergen exposure. In the future, the identification of fungal species using gene sequencing method would help to reveal more fungal species composition and richness as well from a semi-rural area of India. Still, the quantification of fungal spores may not reveal allergy development in all subjects. Hence, the serological detection and identification of major allergens of the culturable fungi would help to reduce the health risk through proper medication.
References
Adams RI, Miletto M, Taylor JW, Bruns TD (2013) Dispersal in microbes: fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J 7:1262. https://doi.org/10.1038/ismej.2013.28
Adams RI, Miletto M, Taylor JW, Bruns TD (2013) The diversity and distribution of fungi on residential surfaces. PLoS ONE 8:78866. https://doi.org/10.1371/journal.pone.0078866
Adhikari A, Sahu S, Bandyopadhyay A, Blanc PD, Moitra S (2015) Fungal contamination of the respiratory tract and associated respiratory impairment among sawmill workers in India. ERJ Open Res 1:00023–2015. https://doi.org/10.1183/23120541.00023-2015
Adhikari A, Sen MM, Gupta-Bhattacharya S, Chanda S (2000) Incidence of allergenically significant fungal aerosol in a rural bakery of West Bengal, India. Mycopathologia 149:35–45. https://doi.org/10.1023/A:1007171420410
Adhikari A, Sen MM, Gupta-Bhattacharya S, Chanda S (2004) Volumetric assessment of airborne fungi in two sections of a rural indoor dairy cattle shed. Environ Int 29:1071–1078. https://doi.org/10.1016/S0160-4120(03)00103-X
Adhikari A, Sen MM, Gupta-Bhattacharya S, Chanda S (2004) Airborne viable, non-viable, and allergenic fungi in a rural agricultural area of India: a 2-year study at five outdoor sampling stations. Sci Total Environ 326:123–141. https://doi.org/10.1016/j.scitotenv.2003.12.007
Alex D, Li D, Calderone R, Peters SM (2013) Identification of Curvularialunata by polymerase chain reaction in a case of fungal endophthalmitis. Med Mycol Case Rep 2:137–140. https://doi.org/10.1016/j.mmcr.2013.07.001
Almaguer M, Aira MJ, Rodríguez-Rajo FJ, Rojas TI (2014) Temporal dynamics of airborne fungi in Havana (Cuba) during dry and rainy seasons: influence of meteorological parameters. Int J Biometeorol 58:1459–1470. https://doi.org/10.1007/s00484-013-0748-6
Alves C, Duarte M, Ferreira M, Alves A, Almeida A, Cunha A (2016) Air quality in a school with dampness and mould problems. Air Qual Atmos Health 9:107–115. https://doi.org/10.1007/s11869-015-0319-6
Andersen AA (1958) New sampler for the collection, sizing, and enumeration of viable airborne particles. J Bacteriol 76:471
Bünger J, Antlauf-Lammers M, Schulz TG, Westphal GA, Müller MM, Ruhnau P, Hallier E (2000) Health complaints and immunological markers of exposure to bioaerosols among biowaste collectors and compost workers. Occup Environ Med 57:458–464. https://doi.org/10.1136/oem.57.7.458
Burbank AJ, Sood AK, Kesic MJ, Peden DB, Hernandez ML (2017) Environmental determinants of allergy and asthma in early life. J Allergy Clin Immunol 140:1–12. https://doi.org/10.1016/j.jaci.2017.05.010
Bush RK, Portnoy JM (2001) The role and abatement of fungal allergens in allergic diseases. J Allergy Clin Immunol 107:430–440. https://doi.org/10.1067/mai.2001.113669
Busse WW, Lemanske RF Jr, Gern JE (2010) Role of viral respiratory infections in asthma and asthma exacerbations. Lancet 376:826–834. https://doi.org/10.1016/S0140-6736(10)61380-3
Butikofer L, Jones B, Sacchi R, Mangiacotti M, Ji W (2018) A new method for modelling biological invasions from early spread data accounting for anthropogenic dispersal. PLoS ONE. https://doi.org/10.1371/journal.pone.0205591
Chakrabarti HS, Das S, Gupta-Bhattacharya S (2012) Outdoor airborne fungal spora load in a suburb of Kolkata, India: its variation, meteorological determinants, and health impact. Int J Environ Health Res 22:37–50. https://doi.org/10.1080/09603123.2011.588323
Chakraborty P, Gupta-Bhattacharya S, Chanda S (2003) Aeromycoflora of an agricultural farm in West Bengal, India: a five-year study (1994–1999). Grana 42:248–254. https://doi.org/10.1080/00173130310016941
Campbell CK, Johnson EM (2013) Identification of pathogenic fungi. Wiley, London
Crandall SG, Gilbert GS (2017) Meteorological factors associated with abundance of airborne fungal spores over natural vegetation. Atmos Environ 162:87–99. https://doi.org/10.1016/j.atmosenv.2017.05.018
Damialis A, Kaimakamis E, Konoglou M, Akritidis I, Traidl-Hoffmann C, Gioulekas D (2017) Estimating the abundance of airborne pollen and fungal spores at variable elevations using an aircraft: how high can they fly? Sci Rep 7:44535. https://doi.org/10.1038/srep44535
Das S, Gupta-Bhattacharya S (2008) Enumerating outdoor aeromycota in suburban West Bengal, India, with reference to respiratory allergy and meteorological factors. Ann Agric Environ Med 15:105–112
Docampo S, Trigo MM, Recio M, Melgar M, García-Sánchez J, Cabezudo B (2011) Fungal spore content of the atmosphere of the Cave of Nerja (southern Spain): diversity and origin. Sci Total Environ 409:835–843. https://doi.org/10.1016/j.scitotenv.2010.10.048
Du P, Du R, Ren W, Lu Z, Fu P (2018) Seasonal variation characteristic of inhalable microbial communities in PM2. 5 in Beijing city, China. Sci Total Environ 610:308–315. https://doi.org/10.1016/j.scitotenv.2017.07.097
Du P, Du R, Ren W, Lu Z, Zhang Y, Fu P (2018) Variations of bacteris and fungi in PM2. 5 in Beijing, China. Atmos Environ 172:55–64. https://doi.org/10.1016/j.atmosenv.2017.10.048
Fernández-Rodríguez S, Tormo-Molina R, Lemonis N, Clot B, O'connor DJ, and Sodeau JR (2018) Comparison of fungal spores concentrations measured with wideband integrated bioaerosol sensor and Hirst methodology. Atmos Environ 175:1–14. https://doi.org/10.1016/j.atmosenv.2017.11.038
Gao M, Jia R, Qiu T, Han M, Song Y, Wang X (2015) Seasonal size distribution of airborne culturable bacteria and fungi and preliminary estimation of their deposition in human lungs during non-haze and haze days. Atmos Environ 118:203–210. https://doi.org/10.1016/j.atmosenv.2015.08.004
Garaga R, Avinash CKR, Kota SH (2019) Seasonal variation of airborne allergenic fungal spores in ambient PM 10—a study in Guwahati, the largest city of north-east India. Air Qual Atmos Health 12:11–20. https://doi.org/10.1007/s11869-018-0624-y
Gent JF, Kezik JM, Hill ME, Tsai E, Li DW, Leaderer BP (2012) Household mold and dust allergens: exposure, sensitization and childhood asthma morbidity. Environ Res 118:86–93. https://doi.org/10.1016/j.envres.2012.07.005
Ghosh D, Chakraborty P, Gupta J, Biswas A, Roy I, Das S, Gupta-Bhattacharya S (2012) Associations between pollen counts, pollutants, and asthma-related hospital admissions in a high-density Indian metropolis. J Asthma 49:792–799. https://doi.org/10.3109/02770903.2012.716473
Goh KJ, Yii ACA, Lapperre TS, Chan AK, Chew FT, Chotirmall SH, Koh MS (2017) Sensitization to Aspergillus species is associated with frequent exacerbations in severe asthma. J Asthma Allergy 10:131. https://doi.org/10.2147/JAA.S130459
Green BJ, Mitakakis TZ, Tovey ER (2003) Allergen detection from 11 fungal species before and after germination. J Allergy Clin Immunol 111:285–289. https://doi.org/10.1067/mai.2003.57
Heseltine E, Rosen J (eds) (2009) WHO guidelines for indoor air quality: dampness and mould. WHO Regional Office Europe, København
Humbal C, Joshi SK, Trivedi UK, Gautam S (2019) Evaluating the colonization and distribution of fungal and bacterial bio-aerosol in Rajkot, western India using multi-proxy approach. Air Qual Atmos Health 12:693–704. https://doi.org/10.1007/s11869-019-00689-6
Hwang SH, Cho JH (2016) Evaluation of airborne fungi and the effects of a platform screen door and station depth in 25 underground subway stations in Seoul, South Korea. Air Qual Atmos Health 9:561–568. https://doi.org/10.1007/s11869-015-0361-4
Jones AM, Harrison RM (2004) The effects of meteorological factors on atmospheric bioaerosol concentrations—a review. Sci Total Environ 326:151–180. https://doi.org/10.1016/j.scitotenv.2003.11.021
Kakde UB (2012) Fungal bioaerosols: global diversity, distribution and its impact on human beings and agricultural crops. Bionano Front 5:323–329
Kemen E, Kemen AC, Rafiqi M, Hempel U, Mendgen K, Hahn M, Voegele RT (2005) Identification of a protein from rust fungi transferred from haustoria into infected plant cells. Mol Plant Microbe Interact 18:1130–1139. https://doi.org/10.1094/MPMI-18-1130
Khan AJ, Deadman ML, Al-Maqbali YM, Al-Sabahi J, Srikandakumar A, Rizvi SG (2001) Biochemical changes in sorghum leaves infected with leaf spot pathogen, Drechslerasorghicola. Plant Pathol J 17:342–346
Kidd SE, Bach PJ, Hingston AO, Mak S, Chow Y, MacDougall L, Kronstad JW, Bartlett KH (2007) Cryptococcus gattii dispersal mechanisms, British Columbia, Canada. Emerg Infect Dis 13:51. https://doi.org/10.3201/eid1301.060823
Kulmala M, Asmi A, Pirjola L (1999) Indoor air aerosol model: the effect of outdoor air, filtration and ventilation on indoor concentrations. Atmos Environ 33:2133–2144. https://doi.org/10.1016/S1352-2310(99)00070-9
Kusai NA, Azmi MMZ, Zulkifly S, Yusof MT, Zainudin NAIM (2016) Morphological and molecular characterization of Curvularia and related species associated with leaf spot disease of rice in Peninsular Malaysia. Rendiconti Lincei 27:205–214. https://doi.org/10.1007/s12210-015-0458-6
Lacey J (1996) Spore dispersal—its role in ecology and disease: the British contribution to fungal aerobiology. Mycol Res 100:641–660. https://doi.org/10.1016/S0953-7562(96)80194-8
Lacey J, Crook B (1988) Fungal and actinomycete spores as pollutants of the workplace and occupational allergens. Ann Occup Hyg 32:515–533. https://doi.org/10.1093/annhyg/32.4.515
Lee T, Grinshpun SA, Martuzevicius D, Adhikari A, Crawford CM, Reponen T (2006) Culturability and concentration of indoor and outdoor airborne fungi in six single-family homes. Atmos Environ 40:2902–2910. https://doi.org/10.1016/j.atmosenv.2006.01.011
Lin WR, Chen YH, Lee MF, Hsu LY, Tien CJ, Shih FM, Hsiao SC, Wang PH (2016) Does spore count matter in fungal allergy?: The role of allergenic fungal species. Allergy Asthma Immunol Res 8:404–411. https://doi.org/10.4168/aair.2016.8.5.404
Loyd AL, Held BW, Linder ER, Smith JA, Blanchette RA (2018) Elucidating wood decomposition by four species of Ganoderma from the United States. Fungal Biol 122:254–263. https://doi.org/10.1016/j.funbio.2018.01.006
Madureira J, Aguiar L, Pereira C, Mendes A, Querido MM, Neves P, Teixeira JP (2018) Indoor exposure to bioaerosol particles: levels and implications for inhalation dose rates in schoolchildren. Air Qual Atmos Health 11:955–964. https://doi.org/10.1007/s11869-018-0599-8
Masaki K, Fukunaga K, Matsusaka M, Kabata H, Tanosaki T, Mochimaru T, Kamatani T, Ohtsuka K, Baba R, Ueda S, Suzuki Y (2017) Characteristics of severe asthma with fungal sensitization. Ann Allergy Asthma Immunol 119:253–257. https://doi.org/10.1016/j.anai.2017.07.008
Mehta D, Dagar A, Kishan J, Singh P, Nehra T, Sharma H (2018) Common allergens prevalent in and around Ambala, Haryana: An intradermal study among patients with asthma and allergic rhinitis and atopic dermatitis. Indian J Dermatol 63:311. https://doi.org/10.4103/ijd.IJD-438-17
Mendell MJ, Mirer AG, Cheung K, Tong M, Douwes J (2011) Respiratory and allergic health effects of dampness, mold, and dampness-related agents: a review of the epidemiologic evidence. Environ Health Perspect 119:748–756. https://doi.org/10.1289/ehp.1002410
Motta O, Zarrella I, Cucciniello R, Capunzo M, De Caro F (2018) A new strategy to control the proliferation of microorganisms in solid hospital waste and the diffusion of nosocomial infections. Infez Med 26:210–215
Nirmalkar J, Deshmukh DK, Deb MK, Tsai YI, Sopajaree K (2015) Mass loading and episodic variation of molecular markers in PM 2.5 aerosols over a rural area in eastern central India. Atmos Environ 117:41–50. https://doi.org/10.1016/j.atmosenv.2015.07.003
Nirmalkar J, Deb MK, Deshmukh DK, Tsai YI, Verma SK (2015) Molecular markers in ambient aerosol in the Mahanadi Riverside Basin of eastern central India during winter. Environ Sci Pollut Res 22:1220–1231. https://doi.org/10.1007/s11356-014-3416-4
Nirmalkar J, Deshmukh DK, Deb MK, Tsai YI, Pervez S (2019) Characteristics of aerosol during major biomass burning events over eastern central India in winter: a tracer-based approach. Atmos Pollut Res 10:817–826. https://doi.org/10.1016/j.apr.2018.12.010
Oliveira M, Ribeiro H, Delgado JL, Abreu I (2009) Seasonal and intradiurnal variation of allergenic fungal spores in urban and rural areas of the North of Portugal. Aerobiologia 25:85–98. https://doi.org/10.1007/s10453-009-9112-z
Pace L, Boccacci L, Casilli M, Fattorini S (2019) Temporal variations in the diversity of airborne fungal spores in a Mediterranean high altitude site. Atmos Environ 210:166–170. https://doi.org/10.1016/j.atmosenv.2019.04.059
Priyamvada H, Singh RK, Akila M, Ravikrishna R, Verma RS, Gunthe SS (2017) Seasonal variation of the dominant allergenic fungal aerosols-one year study from southern Indian region. Sci Rep 7:11171. https://doi.org/10.1038/s41598-017-11727-7
Schenker MB (2005) Farming and asthma. BMJ J. https://doi.org/10.1136/oem.2004.019109
Sharma GPRR (2010) Influence of culture media on growth, colony character and sporulation of fungi isolated from decaying vegetable wastes. J Yeast Fungal Res 1:157–164
Simon-Nobbe B, Denk U, Pöll V, Rid R, Breitenbach M (2008) The spectrum of fungal allergy. Int Arch Allergy Immunol 145:58–86. https://doi.org/10.1159/000107578
Singh AB, Shahi S (2008) Aeroallergens in clinical practice of allergy in India-ARIA Asia Pacific work-shop report. Asian Pac J Allergy Immunol 26:245
Singh AB, Singh A, Pandit T (1999) Respiratory diseases among agricultural industry workers in India: a cross-sectional epidemiological study. Ann Agric Environ Med 6:115–126
Tham R, Vicendese D, Dharmage SC, Hyndman RJ, Newbigin E, Lewis E, O'Sullivan M, Lowe AJ, Taylor P, Bardin P, Tang ML (2017) Associations between outdoor fungal spores and childhood and adolescent asthma hospitalizations. J Allergy Clin Immunol 139:1140–1147. https://doi.org/10.1016/j.jaci.2016.06.046
Tian X, Yang T, He J, Chu Q, Jia X, Huang J (2017) Fungal community and cellulose-degrading genes in the composting process of Chinese medicinal herbal residues. Bioresour Technol 241:374–383. https://doi.org/10.1016/j.biortech.2017.05.116
Tracy MC, Okorie CU, Foley EA, Moss RB (2016) Allergic bronchopulmonary aspergillosis. J Fungi 2:17. https://doi.org/10.3390/jof2020017
Verma PR (2016) White rust of crucifers: an overview of research progress. J Oilseed Brassica 1:78–87
Wang H, Reponen T, Adhikari A, Grinshpun SA (2013) Contribution of fungal spores to organic carbon aerosol in indoor and outdoor environments in the greater Cincinnati area. Aerosol Air Qual Res 13:1348–1355. https://doi.org/10.4209/aaqr.2012.10.0291
Wang X, Liu W, Huang C, Cai J, Shen L, Zou Z, Lu R, Chang J, Wei X, Sun C, Zhao Z (2016) Associations of dwelling characteristics, home dampness, and lifestyle behaviors with indoor airborne culturable fungi: on-site inspection in 454 Shanghai residences. Buil Environ 102:159–166. https://doi.org/10.1016/j.buildenv.2016.03.010
Zhu C, Kawamura K, Kunwar B (2015) Organic tracers of primary biological aerosol particles at subtropical Okinawa Island in the western North Pacific Rim. J Geophys Res Atmos 120:5504–5523. https://doi.org/10.1002/2015JD023611
Acknowledgments
The authors are thankful to the Director, Bose Institute, for giving us permission to do such work. The authors would like to gratefully acknowledge Habra State General Hospital for providing patients’ data with their written consent and clinical support, respectively. United Bank of India, Sree Chaitanya college branch, Habra; Sree Chaitanya College, Habra; Prafullanagar Boys High School, Habra; Jupiter Sawmill house, Habra; and Madhab das’s crop field, Habra, are also acknowledged for granting the permission to sample. Thanks are also due to Mr. Chanchal Chakraborty, Mr. Soumyo Subhra Gupta and Mr. Ashish Kumar Bera for their technical assistance.
Funding
This study was funded by Council of Scientific and Industrial Research (CSIR), New Delhi, India [Grant No.:38(1372)/13/EMR-II].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics declaration
The entire work is approved by Habra State General Hospital’s ethical committee, Habra, West Bengal, India.
Human and animals rights
Yes.
Informed consent
Yes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Karmakar, B., SenGupta, K., Kaur, A. et al. Fungal bio-aerosol in multiple micro-environments from eastern India: source, distribution, and health hazards. SN Appl. Sci. 2, 565 (2020). https://doi.org/10.1007/s42452-020-2323-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2323-1