Abstract
Herein, in order to achieve visible light responsive ability and efficient photogenerated electron–hole pairs separation Zn(1−2x)CuxIn2S(4−1.5x) photocatalysts were fabricated through a simple one-step solvothermal method. The effects of the Cu/Zn molar ratios on the crystal structure, morphology, optical property, as well as photocatalytic activity have been investigated in detail. In addition, the stability of the photocatalysts and apparent quantum yield were measured. The band gap became wider due to the decrease in Cu doping and Zn. The band gap of Zn(1−2x)CuxIn2S(4−1.5x) (x = 0 ~ 0.20) decreased from 2.67 to 1.76 eV with the increase in x value. When Pt was used as a co-catalyst, H2 evolution rates up to 2400 μmol g−1 h−1 were achieved over Zn0.74Cu0.13In2S3.805. Furthermore, when PdS was used as a co-catalyst, the hydrogen generation rates were improved with up to 3600 μmol g−1 h−1 by optimizing the Zn(1−2x)CuxIn2S(4−1.5x). The apparent quantum yield achieved 16% at 420 nm, 9.4% at 500 nm and 9.1% at 600 nm when x = 0.20. The improved visible light photocatalytic performance is considered to be due to the manipulation of the band structure and efficient photogenerated charge separation due to the effects of Cu doping and composition ratio.
Similar content being viewed by others
1 Introduction
Metal sulfide photocatalysts compared to the metal oxide photocatalysts have a advantageous band structure for photocatalytic hydrogen evolution under visible light [1,2,3,4,5,6]. Among the various metal sulfide photocatalysts, the ternary metal chalcogenide ZnIn2S4 may be superior to other sulfides due to low toxicity, good absorption of visible light and stability during photocatalysis [7,8,9,10]. However, pure ZnIn2S4 has limited enhancement of photocatalytic hydrogen production under visible light due to high recombination rate of photogenerated electron–hole pairs and optical absorption range of about λ < 500 nm [11, 12]. In order to solve these drawbacks and enhance the photocatalytic hydrogen production ability, heterojunction strategies between various materials and ZnIn2S4 and metal doping to ZnIn2S4 were investigated. [13,14,15,16,17]. Yang and co-researchers have reported Cu and Ga co-doped ZnIn2S4 for enhanced photocatalytic hydrogen generation activity [17]. CuInS2 is a ternary chalcogenide semiconductor with a band gap and high absorption coefficient that is well matched to the AM0 or AM1.5 solar spectrum. Therefore, combination with CuInS2 and ZnIn2S4 was investigated and a high hydrogen generation capacity was revealed [18,19,20]. In particular, the lattice between CuInS2 and ZnIn2S4 is better matched by similar sulfides compared to other coupled photocatalysts [21]. Indium sulfide (In2S3) is expected as candidate for optoelectronic and photovoltaic materials because of its low toxicity, high carrier mobility and stability in photocatalytic reactions [22, 23]. Song et al. showed improved hydrogen-producing activity of ZnIn2S4/In2S3 prepared by CTAB-assisted hydrothermal method with excess indium and found optimum content of indium [23]. It is generally reported that an excess or deficiency of the doping amount is disadvantageous to the photocatalytic activity [13, 14, 17]. Therefore, investigation of the optimal doping amount is necessary for further improvement of photocatalytic activity. In this study, adjustment of the composition ratio and co-catalyst was investigated to further improve the photocatalytic activity of a series of Zn(1−2x)CuxIn2S(4−1.5x) (x = 0, 0.03, 0.06, 0.10, 0.13, 0.20) photocatalysts doped with different amounts of Cu and excess In.
2 Experiment
2.1 Preparation of photocatalysts
In this experiment, all chemicals were of analytical purity and used without further purification. The Zn(1−2x)CuxIn2S(4−1.5x) (x = 0, 0.01, 0.03, 0.06, 0.10, 0.13, 0.20) photocatalysts were prepared by previous reported CTAB-assisted one-step hydrothermal method [24]. Cetyltrimethylammonium bromide (CTAB, 3.76 mmol) (Wako Pure Chemical Industries, Osaka, Japan), stoichiometric moles of ZnSO4 7H2O (Nacalai Tesque, Kyoto, Japan), InCl3·4H2O, CuCl (I) and excess thioacetamide (TAA, 8.00 mmol) (Wako Pure Chemical Industries, Ltd., Osaka, Japan) were dissolved in 50 mL of distilled water under stirring. Then, to remove dissolved oxygen and keep the added Cu monovalent, the mixture solution was purged with nitrogen for 10 min. The mixture was added into a 100 mL Teflon autoclave. The autoclave was sealed and held at 160 °C for 1 h and naturally cooled to room temperature. After cooling, the products were washed with ethanol and distilled water few times, respectively, then vacuum-dried at 40 °C for 4 h and ground for 30 min. ZnIn2S4 was prepared in the same method as the reference material.
2.2 Characterization of samples
Crystalline phases of the photocatalysts were decided from X-ray diffraction (XRD) patterns measured a Rigaku RINT Ultima-IV diffractometer. It was measured by scanning rate of 0.04°/s and employing Cu radiation source. Crystallite size was determined using Scherrer’s Eq. (1).
where D, K, λ, B and θ represent crystallite size, the shape factor (0.9), Cu Kα wavelength (0.154056 nm), full width at half maximum intensity in radians and Bragg’s diffraction angle, respectively. To determine the binding state of the contained elements and the position of valence band (VBXPS), X-ray photoelectron spectroscopy (XPS) measurements were obtained on a PHI Quantera SXM photoelectron spectrometer using Al Kα radiation source. Elemental mapping of prepared photocatalyst was determined by electron probe microanalysis (EPMA) with JXA-8530F. Binding energy was corrected using the C1s peak at 284 eV as a reference for surface charge effects. Surface condition of samples was observed using scanning electron microscope (SEM) with a Hitachi S-4000. Also, internal structure was observed by the transmission electron microscopy (TEM) on JEOL JEM1011. UV–Vis diffuse reflectance spectra (DRS) were measured using Shimadzu UV-2450 spectrophotometer equipped with an integrating sphere attachment and BaSO4 as a reference standard. Using a Shimadzu RF-5300PC, photoluminescence (PL) spectra of each photocatalyst were detected with an excitation wavelength of 350 nm. To calculate the band gap energy, a classical extrapolation approach was using Eq. (2), and the positions of the valence band and the conduction band were estimated from VBXPS and band gap energy measurement results.
where hν, α, A and Eg represent the discrete photon energy, optical absorption coefficient, Planck’s constant and photonic energy band gap, and n = 1/2 for direct band gap semiconductor [25].
2.3 Photocatalytic hydrogen generation experiment
The photocatalytic hydrogen generation experiment was tested in the 123-mL Pyrex reactor. A 300 W xenon lamp was employed as the side irradiation light source equipped with cutoff filter (λ ≥ 420 nm) due to remove UV part. In all experiments, 40 mL (pH 12) of solution containing 40 mg of catalyst, 10 mL of 0.04 ppm co-catalyst solution (1 wt%) and 0.25 M Na2SO3/0.35 M Na2S mixed sacrificial agent was added into the reaction cell. The light source was a 4000–4500 µW/cm2 Xe lamp for 6 h, with a cutoff filter (λ ≥ 420 nm). The co-catalysts were loaded onto the photocatalyst by dropping a 1000 ppm standard solution into the reaction vessel and irradiating with visible light. Nitrogen purged through the system for 30 min before reaction to remove oxygen. The concentrations of H2 were measured using gas chromatograph system (GC) consisting of injection, column and thermal conductivity detector (TCD), of which temperatures were kept at 50 °C. The hydrogen generation experimental conditions are shown in Table 1. On the stability of the photocatalyst, one cycle of photocatalytic hydrogen generation was 6 h, and 4 cycles were performed under the same conditions. At the end of each cycle, a nitrogen purge was performed in the reaction vessel to remove residual hydrogen. Apparent quantum yield was measured using band-pass filter (420 nm, 500 nm and 600 nm) and calculated using Eq. (3).
3 Results and discussion
3.1 Photocatalytic activity
For optimal co-catalyst studies, Rh, Ru, Pt, or PdS was loaded on Zn0.74Cu0.13In2S3.905 and compared hydrogen generation rates. The result is shown in Fig. S1. The hydrogen production activity of the photocatalyst loaded with each co-catalyst improved in the order of Rh < Ru < Pt < PdS.
Figure 1 shows the effect of Cu and excess In doping amount was studied with Zn(1−2x)CuxIn2S(4−1.5x) (x = 0, 0.01, 0.03, 0.06, 0.10, 0.13 and 0.20). The hydrogen production activity of each Zn(1−2x)CuxIn2S(4−1.5x) catalyst was great in the order of x = 0 < x = 0.01 < x = 0.13 < x = 0.20 < x = 0.10 < x = 0.06 < x = 0.03. The hydrogen generation rate of Zn0.94Cu0.03In2S3.955 was the highest, reaching about 3600 μmol g −1 h −1. This result is about four times as high as 900 μmol g −1 h −1 of the hydrogen production rate of pure ZnIn2S4 using PdS as co-catalyst. This difference in hydrogen generation activity is considered to be due to the increase in absorption wavelength due to the incorporation of CuInS2 and In2S3 into ZnIn2S4. Thus, the higher hydrogen generation rate of x = 0.03 than x = 0 is largely related to the absorption of light with a wavelength of 400 nm to 600 nm from result of DRS. Photocatalytic H2 production of recent works on metal chalcogenide photocatalysts is given in Table S1.
The stability of the photocatalytic activity is also important to obtain a stable amount of hydrogen production. In order to research stability, Zn0.94Cu0.03In2S3.955 loaded PdS 1.0 wt% under visible light irradiation was performed in a time-dependent photocatalytic hydrogen production cycle experiment, as shown in Fig. 2. The hydrogen generation activity of Zn0.94Cu0.03In2S3.955 did not decrease significantly in the second cycle. However, after the third cycles, the catalytic activity of hydrogen production decreased to some extent. This phenomenon may have resulted in slight deterioration of the photocatalyst, because of the long-time photocatalytic hydrogen production reaction process.
As we investigate the cause of enhanced photocatalytic ability, the apparent quantum yield in the visible region was investigated. Table S2 shows an apparent quantum yield when a xenon lamp irradiated to a Zn0.94Cu0.03In2S3.955 and Zn0.60Cu0.20In2S3.70 photocatalysts through a band-pass filter which passes wavelengths of only 420 nm, 500 nm and 600 nm. The apparent quantum yield was higher at Zn0.94Cu0.03In2S3.955 than at Zn0.60Cu0.20In2S3.70 in the short wavelength (λ = 420 nm and 500 nm). Conversely, in the long wavelength (λ = 600 nm), the apparent quantum yield showed a higher at Zn0.60Cu0.20In2S3.70. These result were consistent with the measurement results of DRS and suggest that the improvement of the hydrogen production activity of Zn(1−2x)CuxIn2S(4−1.5x) photocatalyst is mainly due to the light absorption ability.
3.2 Structural characterization
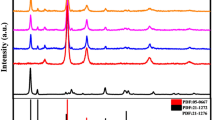
Figure 3 shows XRD pattern measurements of prepared ZnIn2S4, Zn0.94Cu0.03In2S3.955, Zn0.74Cu0.13In2S3.805 and Zn0.60Cu0.20In2S3.7. The XRD pattern of ZnIn2S4, Zn0.94Cu0.03In2S3.955, Zn0.74Cu0.13In2S3.805 and Zn0.60Cu0.20In2S3.7 can be indexed as a hexagonal ZnIn2S4 structure (JCPDS No. 65-2023). These XRD patterns are consistent with those reported in previous studies [26], and Zn(1−2x) CuxIn2S(4−1.5x) has a hexagonal structure regardless of the amount of doping and is similar to ZnS. It became clear that it contained almost no impurities. No peaks derived from Cu and excess In were observed. This is due to a very small amount of doping. Furthermore, with respect to the (006) plane peak, Cu doping and excess In cause a slight shift to high angles. This means that the planar spacing has been reduced by doping, suggesting that Cu and excess In may be incorporated into the ZnIn2S4 crystal structure and exist as a solid solution. The crystallite sizes calculated using Scherrer’s equation are listed in Table S3. Crystallite size decreased slightly with increasing Cu and excess In doping.
In order to confirm Cu and Pd element, the Zn0.60Cu0.20In2S3.7 was research by XPS. The results are shown in Fig. 4. Also, Table S4 shows the element ratios of each photocatalytic catalyst determined from the XPS spectrum. Figure S2 shows the survey spectra before and after the hydrogen production are almost same. In the narrow spectrum, Zn 2p spectrum shows two peaks at the binding energies of 1020.4 eV and 1043.6 eV. The two peaks are indexed Zn2p3/2 and Zn 2p1/2, respectively. The positions of peaks at 452.7 eV and 445.1 eV can be belonging to In 2p3/2 and In 2p5/2, and the positions of peaks at 161.3 eV and 162.6 eV peaks for S 2p are indexed at S 2p3/2 and S 2p1/2, which attribute to ZnIn2S4, In2S3 and CuInS2, respectively. Cu 2p3/2 peaks positioned at 932 eV indicate that Cu is monovalent, and the presence of CuInS2 [27, 28]. Also, Pd peak is not observed before the hydrogen production experiment, but after the experiment Pd presence can be confirmed. This XPS result indicates that Pd employed was deposited as PdS on the catalyst. Furthermore, elemental mapping using EPMA measurements showed that Zn, In, S and Cu were uniformly present in the photocatalyst particles (Fig. S3).
3.3 Morphological analysis
The morphology of as-synthesized Zn(1−2x)CuxIn2S(1−1.5x) (x = 0, 0.03, 0.06, 0.10, 0.13, 0.20) photocatalyst was characterized by SEM and TEM. Figure 5a shows the typical petal-microsphere ZnIn2S4 with folding the nanosheet [24, 30]. Figures 5b and 4c show increasing the doping amount of Cu and excess In broke the spherical structure. The nanosheet structure is obvious up to x = 0.06. However, when values of x higher than that, the nanosheet structure was agglomerated. The difference of morphology between x = 0 with x = 0.10, 0.13 and 0.20 was clear. It suggests that the solid solution was formed by doping Cu and excess In. The TEM images of prepared Zn(1−2x)CuxIn2S(4−1.5x) photocatalysts are shown in Fig. S4. At ZnIn2S4, several thin lines were observed. It suggests a petal-microsphere structure with nanosheets. When x = 0.03 and 0.06, microsphere was not observed and nanosheets were reduced clearly. When x = 0.10, 0.13 and 0.20, the nanosheets were almost unobservable and almost no transmission. This result suggests doping Cu and excess In broke morphology. It matched the SEM images. The mechanism of change shape is still unclear at present and requires further researches.
3.4 Optical analysis
The effects of Cu and excess In doping amount to the absorption region in Zn(1−2x)CuxIn2S(4−1.5x) (x = 0, 0.03, 0.06, 0.10, 0.13 and 0.20) were investigated by UV–Vis diffuse reflectance spectra (DRS) in Fig. 6. Tauc plot obtained from the UV–Vis DRS spectrum for estimating band gap energy is shown in Fig. S5.
As shown in the results of DRS and Tauc plot, the absorption edge of ZnIn2S4 without doping was the shortest of the other samples, less than 500 nm, and the calculated the band gap energy was about 2.67 eV. On the other hand, the absorption edge of the excess In- and Cu-doped Zn(1−2x)CuxIn2S(4−1.5x) photocatalysts had more red-shift with an increase in the doping amount. The absorption edge of Zn0.60Cu0.20In2S3.7 reached up to 770 nm. The absorbance of the Zn(1−2x)CuxIn2S(4−1.5x) (x = 0.03 and 0.06) photocatalysts was higher than that of the Zn(1−2x)CuxIn2S(4−1.5x) (x = 0.10, 0.13 and 0.20) at wavelengths of less than 550 nm. As well, the band gap energy decreased as the amount of excess In and Cu doping increasing. The band gap energy of Zn0.60Cu0.20In2S3.7 photocatalyst decreases up to 1.98 eV.
In order to further research the band structure of photocatalyst, valence band edge was measured for the prepared photocatalysts by XPS. Figure S6 shows that the doping of Cu and excess In shifted the valence band edge to the negative side. This result was consistent with previous studies which show that Cu+ doping inserts Cu 3d orbital into the ZnIn2S4 valence band and shifts it to the negative side [12, 22]. The significant decrease in band gap energy size compared to the valence band shift level indicates that the conduction band is positively shifted. The conduction band shifting effect is due to excess In doping. It was reported that the inserting of In3+ constitutes a sub-band on the positive side than the conduction band of ZnIn2S4 [31].
Photoluminescence spectrum was recorded for ZnIn2S4, Zn0.94Cu0.03In2S3.955, Zn0.74Cu0.13In2S3.805 and Zn0.60Cu0.20In2S0.37 due to investigate the effect of the doping amount of Cu and excess indium to the recombination of photogenerated electron/hole pairs in photocatalyst. Generally, the stronger PL intensity suggests the high recombination rate of photogenerated carriers. And as shown in Fig. 7, pure ZnIn2S4 has a broad emission peak of 400–700 nm, which represents multiple recombination centers [32, 33]. The peak intensity of Zn0.94Cu0.03In2S3.955 decreased dramatically compared to ZnIn2S4, and that of Zn0.74Cu0.13In2S3.805 further decreased. However, intensity of Zn0.60Cu0.20In2S0.37 increased dramatically compared to Zn0.74Cu0.13In2S3.805. This phenomenon is consistent with the results that reported [26]. This suggests that low Cu and excessive In doping can effectively delay the recombination of electron/hole pairs, but high doping promotes recombination.
3.5 Proposed hydrogenation mechanism
The reaction mechanism based on the band gap of Zn0.94Cu0.03In2S3.955 loaded PdS 1.0 wt% is shown in Fig. 8. The valence band edge of pure ZnIn2S4 is composed of S3p + Zn3d, and the conduction band edge is composed of In5s5p + Zn4s4p [17]. Regarding the conduction band of Zn(1−2x)CuxIn2S(4−1.5x), the ratio of In2S3 increases by lowering the ratio of Zn in ZnIn2S4, and the effect of the In5s5p electron orbit derived from In2S3 becomes large, so the conduction band is considered to shift to the positive position. The valence band of Zn(1−2x)CuxIn2S(4−1.5x) is considered to be that, by doping Cu into ZnIn2S4, Cu3d is added to S3p + Zn3d, and the valence band shifts to the negative position. Figure S7 shows the Zn(1−2x)CuxIn2S(4−1.5x) (x = 0, 0.03, 0.06, 0.10, 0.20) band structure considered from the valence band edge determined by XPS and the band gap energy determined from DRS. The doping of Cu and excess In has the advantage of enables reaction under longer wavelength light, delaying the recombination of photogenerated electron–hole pairs. On the other hand, high doping amount promotes recombination of photogenerated electron–hole pairs. In addition, excess In doping has the disadvantage of lowering the CB potential and lowering the excess potential for water splitting [26]. Therefore, Zn0.94Cu0.03In2S3.955 was suggested experimentally the best catalyst with a balance between these two factors.
4 Conclusion
The Zn(1−2x)CuxIn2S(4−1.5x) photocatalyst was synthesized in situ by a one-pot solvothermal reaction. The Zn(1−2x)CuxIn2S(4−1.5x) photocatalyst significantly improves the photocatalytic performance of ZnIn2S4 for H2 evolution under visible light illumination.
The Zn(1−2x)CuxIn2S(4−1.5x) photocatalyst is considered to be composed of a ZnIn2S4-CuInS2-In2S3 composite catalysts from the XPS results. In addition, ZnIn2S4, CuInS2 and In2S3 may be partially in solid solution from XRD results, elemental mapping using EPMA and SEM images.
The Zn(1−2x)CuxIn2S(4−1.5x) heterostructure photocatalyst extends the light absorption wavelength range of ZnIn2S4 and significantly improves the photocatalytic performance for H2 generation under visible light illumination. The hydrogen generation activity was improved to 3600 μmol g−1 h−1 with the optimum Zn(1−2x)CuxIn2S(4−1.5x) system. On the other hand, the apparent quantum yield reached up to 9.1% at a wavelength of 600 nm. The doping of Cu and excess In caused the manipulation of the band structure and the hole electron recombination, which contributed to the photocatalytic activity. These results suggest inspiration for the design of composite photocatalysts with visible and efficient photocatalyst H2 generation.
References
Tsuji I, Kato H, Kudo A (2006) Photocatalytic hydrogen evolution on ZnS–CuInS2–AgInS2 solid solution photocatalysts with wide visible light absorption bands. Chem Mater 18:1969–1975
You D, Pan B, Jiang F, Zhou Y, Su W (2016) CdS nanoparticles/CeO2 nanorods composite with high-efficiency visible-light-driven photocatalytic activity. Appl Surf Sci 363:154–160
Jin X, Chen F, Jia D, Cao Y, Duan H, Long M, Yang I (2019) Influences of synthetic conditions on the photocatalytic performance of ZnS/graphene composites. J Alloys Compd 780:299–305
Swain G, Sultana S, Parida K (2020) Constructing a novel surfactant-free MoS2 nanosheet modified MgIn2S4 marigold microflower: an efficient visible-light driven 2D–2D p–n Heterojunction Photocatalyst Toward HER and pH regulated NRR. ACS Sustain Chem Eng 12:4848–4862
Peng S, An R, Li Y, Lu G, Li S (2012) Remarkable enhancement of photocatalytic hydrogen evolution over Cd0.5Zn0.5S by bismuth-doping. Int J Hydrog Energy 37:1366–1374
Swain G, Sultana S, Moma J, Parida K (2018) Fabrication of hierarchical two-dimensional MoS2 nanoflowers decorated upon cubic CaIn2S4 microflowers: facile approach to construct novel metal-Free p–n heterojunction semiconductors with superior charge separation efficiency. Inorg Chem 57(16):10059–10071
Lei Z, You W, Liu M, Zhou G, Takata T, Hara M, Domen K, Li C (2003) Photocatalytic water reduction under visible light on a novel ZnIn2S4 catalyst synthesized by hydrothermal method. Chem Commun 17:2142–2143
Yang G, Ding H, Chen D, Feng J, Hao Q, Zhu Y (2018) Construction of urchin-like ZnIn2S4–Au–TiO2 heterostructure with enhanced activity for photocatalytic hydrogen evolution. Appl Catal B Environ 234:260–267
Li YX, Hou YL, Fu QY, Peng SQ, Hu YH (2017) Oriented growth of ZnIn2S4/In(OH)3 heterojunction by a facile hydrothermal transformation for efficient photocatalytic H2 production. Appl Catal B Environ 206:726–733
Swain G, Sultana S, Parida K (2019) One-Pot-architectured Au-nanodot-promoted MoS2/ZnIn2S4: a novel p–n heterojunction photocatalyst for enhanced hydrogen production and phenol degradation. Inorg Chem 58(15):9941–9955
Shen S, Zhao L, Zhou Z, Guo L (2008) Enhanced photocatalytic hydrogen evolution over Cu-doped ZnIn2S4 under visible light irradiation. J Phys Chem C 112:16148–16155
Fan B, Chen ZH, Liu Q, Zhang ZG, Fang XM (2016) One-pot hydrothermal synthesis of Ni-doped ZnIn2S4 nanostructured film photoelectrodes with enhanced photoelectrochemical performance. Appl Surf Sci 370:252–259
Li Y, Wang J, Peng S, Liu G, Li S (2010) Photocatalytic hydrogen generation in the presence of glucose over ZnS-coated ZnIn2S4 under visible light irradiation. Int J Hydrog Energy 35:7116–7126
Zeng D, Xiao L, Ong W-J, Wu P, Zheng H, Chen Y, Peng D-L (2017) Hierarchical ZnIn2S4/MoSe2 nanoarchitectures for efficient noble-metal-free photocatalytic hydrogen evolution under visible light. Chemsuschem 10:4624–4631
Xu B, He P, Liu H, Wang P, Zhou G, Wang X (2014) A 1D/2D helical CdS/ZnIn2S4 nano-heterostructure. Angew Chem Int Ed 53:2339–2343
Wang S, Guan BY, Lou XWD (2018) Construction of ZnIn2S4-In2O3 hierarchical tubular heterostructures for efficient CO2 photoreduction. J Am Chem Soc 140:5037–5040
Yang J, Fu H, Yang DF, Gao WL, Cong RH, Yang T (2015) ZnGa2−xInxS4 (0 ≤ x ≤ 0.4) and Zn1−2y(CuGa)yGa1.7In0.3S4 (0.1 ≤ y ≤ 0.2): optimize visible light photocatalytic H2 evolution by fine modulation of band structures. Inorg Chem 54:2467–2473
Nanu M, Schooman J, Goossens (2005) Nanocomposite three-dimensional solar cells obtained by chemical spray deposition. Nano Lett 5:1716
Liu A, Yu C, Lin J, Sun G, Xu G, Huang Y, Liu Z, Tang C (2019) Construction of CuInS2@ZIF-8 nanocomposites with enhanced photocatalytic activity and durability. Mater Res Bull 112:147–153
Aldakov D, Lefrancois A, Reiss P (2013) Ternary and quaternary metal chalcogenide nanocrystals: synthesis, properties and applications. J Phys Chem C 1:3756–3776
Guan Z, Pan J, Li Q, Li G, Yang J (2019) Boosting visible-light photocatalytic hydrogen evolution with an efficient CuInS2/ZnIn2S4 2D/2D heterojunction. ACS Sustain Chem. Eng 7:7736–7742
Han M, Yu Lu, Chen W, Wang W, Jia J (2016) Fabrication and photoelectrochemical characteristics of In2S3 nano-flower films on TiO2 nanorods arrays. Appl Surf Sci 369:108–114
Song K, Zhu R, Tian F, Cao G, Ouyang F (2015) Effects of indium contents on photocatalytic performance of ZnIn2S4 for hydrogen evolution under visible light. J Solid State Chem 232:138–143
Shen SH, Zhao L, Guo LJ (2008) Cetyltrimethylammoniumbromide (CTAB)-assisted hydrothermal synthesis of ZnIn2S4 as an efficient visible-light-driven photocatalyst for hydrogen production. Int J Hydrog Energy 33:4501–4510
Feng C, Yang X, Sun Z, Xue J, Sun L, Wang J, He Z, Yu J (2019) Dual interfacial synergism in Au-Pd/ZnIn2S4 for promoting photocatalytic selective oxidation of aromatic alcohol. Appl Surf Sci 501:144018
Yuan D, Sun M, Tang S, Zhang Y, Wang Z, Qi J, Rao Y, Zhang Q (2020) All-solid-state BiVO4/ZnIn2S4 Z-scheme composite with efficient charge separations for improved visible light photocatalytic organics degradation. Chin Chem Lett 31:547–550
Yue W, Han S, Peng R, Shen W, Geng H, Wu F, Tao S, Wang M (2010) CuInS2 quantum dots synthesized by a solvothermal route and their application as effective electron acceptors for hybrid solar cells. J Mater Chem 20:7570–7578
Cao HY, Deng HM, Zhou WL, Tao JH, Chen LL, Huang L, Sun L, Yue FY, Yang PX, Chu JH (2015) Investigation of microstructural and optical properties of Cu(In, Al)Se2 thin films with various copper content. J Alloy Compd 65:208–213
Tateishi I, Furukawa M, Katsumata H, Kaneco S (2019) The effect of Cu and Ga doped ZnIn2S4 under visible light on the high generation of H2 production. ChemEngineering 3(4):79
Chen S, Li S, Xiong L, Wang G (2018) In-situ growth of ZnIn2S4 decorated on electrospun TiO2 nanofibers with enhanced visible-light photocatalytic activity. Chem Phys Lett 706:68–75
Li Y, Han P, Hou Y, Peng S, Kuang X (2019) Oriented ZnmIn2Sm+3@In2S3 heterojunction with hierarchical structure for efficient photocatalytic hydrogen evolution. Appl Catal B: Environ 244:604–611
Xia Y, Li Q, Lv KL, Tang DG, Li M (2017) Superiority of graphene over carbon analogs for enhanced photocatalytic H2-production activity of ZnIn2S4. Appl Catal B Environ 206:344–352
Zhang Z, Lin H, Zhang J, Wang F, Xie Y, Sheng X, Gu Y, Zhao H, Wang X (2018) In situ constructing interfacial contact MoS2/ZnIn2S4 heterostructure for enhancing solar photocatalytic hydrogen evolution. Appl Catal B 233:112–119
Acknowledgements
The author is grateful to the graduate school department of Mie University for the financial and technical support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tateishi, I., Furukawa, M., Katsumata, H. et al. Efficient photocatalytic hydrogen production by Zn(1−2x)CuxIn2S(4−1.5x) co-doped with Cu and excess in under visible light irradiation. SN Appl. Sci. 2, 1681 (2020). https://doi.org/10.1007/s42452-020-03450-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03450-2