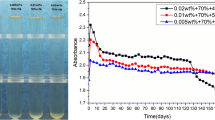
Abstract
It is a very challenging purpose to suspend a nanoparticle in a non-polar medium. In this study, stable nanofluid of nanostructured lamellar boric acid (nBA), which is highly hydrophilic compound (log Kow value is 0.18), to be suspended in non-polar media was successfully prepared. Group III base oil; i.e. mineral base oil, was used for modeling non-polar media. According to characterization examinations, optimal nBA nanofluid adding ratio is found to be 3%. Viscosity analyses were held by Brookfield DV2T® Viscometer. Friction effect of adding the nBA nanofluid into base oil was characterized by a reciprocating tribometer. Stability of nanofluid suspension in base oil was characterized by Turbiscan Tower® instrument. Viscosity of original base oil was decreased by 2.88% at 100 °C, friction coefficient value of base oil was decreased by 2.91%; Turbiscan Stability Index (TSI) values of prepared sample was 2.9. These characterizations showed that boric acid, a highly hydrophobic material, suspended in a non-polar environment with the help of a simple route containing auxiliary chemicals and ultrasonic horn.
Graphic abstract

Similar content being viewed by others
1 Introduction
It is a very challenging purpose to suspend a nanoparticle in a non-polar medium [1, 2]. Nanoparticles have high Gibbs free energy as they have tendencies to form agglomerates, and they are not thermodynamically stable. Thus it is very difficult to obtain such a suspended solution and the current related literature is still immature on this issue [3]. In current literature, researchers have used several methods to disperse and suspend nanomaterials in the non-polar medium. Widely used methods for this purpose are magnetic stirring, chemical agitation, agitation by ultrasonic shaker, agitation using mechanical ball milling, ultrasonic horn, ultrasonic bath, adding surfactant and surface modification of nanoparticles [4,5,6,7]. In this study, we provide the suspension of boric acid in non-polar medium by preparing a stable nanofluid of boric acid. This boric acid nanofluid was prepared by practical and easy methods (by adding chemicals and using ultrasonic horn) without chemical modification. The chemicals that constitute the base liquid of the nanofluid were selected from substances that will help the suspension of boric acid in a non-polar environment. Two-step process method was followed to prepare nBA nanofluid. Two-step process method implies the fact that the nanoparticle is taken as powder and by adding chemical and using a mechanical process nanofluid is obtained as a suspension. On the other hand, one-step method includes preparation of a nanofluid by synthesizing the nanoparticle in dispersing media [1, 2]. Suspension duration of additive nanoparticles in non-polar mediums is also important. According to the available literature, limited studies have been addressed on stable suspension duration [8,9,10,11]. Researchers have also studied to disperse and suspend boric acid particles of different sizes in different media [12,13,14]. However, these studies did not focus on how long boric acid particles were suspended in the corresponding media. In addition, detailed characterization of the suspension of boric acid particles were not addressed in those studies. Moreover, studies did not use the turbiscan instrument for stability characterization.
An application area of boric acid nanofluid may be the use of it as an engine oil additive. Boric acid has found to have superior lubricant characteristics and it is a well-known lubrication additive [15, 16]. Boric acid has a lamellar structure that enables formation of a slippery surface between two contacting pairs [17, 18]. Boric acid also forms tribofilm on sliding surfaces and prevents excessive wear on these surfaces by reducing friction [12]. In addition, boric acid acts as a third body between two contacting surfaces providing hybrid lubrication (oil + particle lubrication) when it is added to engine oil [13, 19]. However, one of the biggest disadvantages of using a nanoparticle as a lubricant additive is that the particulate additives are agglomerated and collapsed in engine oils. In the case of polar engine oil additive nanoparticles (such as boric acid that has log Kow value as 0.18, highly polar), another disadvantage is chemical incompatibility; it is hard to suspend them in non-polar media. Additives should be suspended in engine oils. Agglomeration or sedimentation of nanoparticles in engine oil may result in clogging of micro channels, may decrease thermal conductivity, may change the viscosity of the oil and may disrupt the regular operation of the engine [20].
In view of above, the main purpose of the present study is to prepare nBA nanofluid and keep the nano boric acid suspended in non-polar media (Group III base oil; i.e. mineral base oil), and characterize structure, examine the effect of nBA nanofluid on mineral base oil to viscosity, friction and suspension properties. A new formula for the Nano lamellar Boric Acid (nBA) nanofluid is proposed in the present study. For this purpose, a new additive preparation procedure was developed and novel suspension enhancing chemicals were introduced for the first time. The proposed formula goes beyond the state-of-the-art by using these chemicals and preparation method and presenting longer duration of suspension. Another novel aspect of this study is implementation of suspension characterization in detail by applying a totally new approach [21]. Suspension characterizations were conducted by Turbiscan Tower® instrument that gave scientific detailed information about suspension stability.
2 Materials and methods
2.1 Preparation of nBA nanofluid and nBA nanofluid added mineral base oil
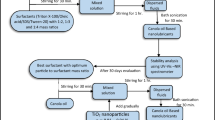
nBA powders were supplied from Tribor, Inc. (Istanbul, Turkey). The TEM micrographs showing the size and general morphology of the powders are shown in Fig. 1. The nano-level lamellar structure stands out in the TEM micrographs of the nBA nanoparticle, thus, this nanoparticle is called Nano-structured lamellar Boric Acid (nBA). Then, Lutensol® XL 80 (a selected type of nonionic surfactant) and nonanol (a fatty alcohol) were obtained from Merck and BASF, respectively. These two chemicals were used to prepare homogenous dispersion of the nBA nanoparticles. Lutensol® XL 80 is alkyl polyethylene glycol ether made from C10-Guerbet Alcohol and ethylene oxide. Second suspension chemical, nonanol, is a fatty alcohol.
In order to prepare the nBA nanofluid, two-step process method was followed. In two-step process method the nanoparticle is taken as powder and by adding chemical and using a mechanical process nanofluid is obtained as a suspension. On the other hand, one-step method makes use of preparation of a nanofluid by synthesizing the nanoparticle in dispersing media [1, 2].
5 g of nBA was weighed and put in a glass vial and then 5 g of Lutensol® XL80 was added to the nBA powder. Ultrasonic horn was applied for 15 min. In this step, Hielscher UP 400St instrument was used at a constant frequency of 24 kHz for 15 min. in 50% amplitude. 90 g nonanol was added to the solution, and after the final addition, the solution was mechanically stirred again for 15 min. These preparation procedures are graphically illustrated in Fig. 2.
Prepared nanofluid was introduced to mineral base oil and stirred mechanically for 15 min at room temperature. Three samples were prepared by different addition ratios of nBA nanofluid, that were 1, 3 and 5 percentages.
2.2 Viscosity analysis of nBA nanofluid added mineral base oil
Viscosities of nBA nanofluid added mineral base oil samples were measured before and after the addition. Brookfield DV2T® Viscometer and ULA0 Spindle was used for characterizations. Test duration is 2 min, for all samples, tests were applied for 3 times. Tests were conducted at 25 °C, 40 °C and 100 °C. Samples were heated to 40 °C and 100 °C then they were tested. Spindle speed is fixed to 25 rpm for 25 °C, 200 rpm for 40 °C and 100 °C.
2.3 nBA nanofluid effect on mineral base oil friction coefficient
Tribometer tests were performed to observe how suspended nBA nanofluid affects the friction coefficient of mineral base oil. For this purpose, a special tribometer (UTS Tribometer T30M-HT® with a reciprocating test module) was used. The tests were performed according to the ASTM G133 using ball-on-plate configuration where an 8 mm ball made of 100Cr6 and a steel plate (12 mm × 15 mm × 3 mm in dimensions) made of hardened 4140 steel with the hardness of 52 HRC were used. The surfaces of the plates used were polished carefully after machining to be able to have mirror-like surfaces. Fresh plate surface was used for each test. For each condition, at least three tests were performed to be sure of repeatable results. Friction tests were conducted with five drops of sample on the plate. The tests were made with 8 mm stroke and 2 Hz frequency for a total distance of 32 m at room temperature.
2.4 Suspension characterization of nBA nanofluid added mineral base oil
Suspension characterization of nBA nanofluid added mineral base oil was conducted by turbidimetry method. In turbidimetry method, intensity of light that scattered or transmitted by suspended nanoparticles in nanofluid is measured. Turbidimetry is the most suitable and the most reliable method to measure the suspension stability of nanoparticles [22,23,24,25,26,27]. To the best of our knowledge, it has not previously been used for the suspension characterization of a nanoparticle in the mineral base oil.
According to Mengual et al. [28], turbiscan instrument detects destabilization (coalescence) 50 times sharper than naked eye. Turbiscan Tower® instrument uses 880 nm light source; after the light beam is passed through the sample cuvette, detectors measure the differences of both Back Scattering (BS) and Transmittance (T) intensities of light over time and record the intensity differences of these values. By following this way, it monitors the stability of samples. Narrower fluctuation in sedimentation profile (at BS and T graphs) means minor changes of T and BS values over time. Minor changes in T and BS refer to less displacement/movement of particles in solution which present better suspension stability. Turbiscan instrument software, namely Formulaction® gives a parameter called Turbiscan Stability Index (TSI). The TSI value is a numeric and unitless value that includes information of T, BS and other parameters about stability. Lower TSI value refers to higher suspension stability.
3 Results and discussion
3.1 Characterization of nBA nanofluid
Figure 3 presents particle size distribution results obtained by means of Zeta Sizer instrument and TEM micrograph of the nBA nanofluid prepared. Size distribution graph (Fig. 3a) with a unimodal trend reveals that there is one nBA particle size that is 175 nm in this solution with an overwhelming ratio of 95%. If the nBA nanoparticles had been agglomerated and formed larger particles, the particle size distribution measurement would also have peaks at higher nm values. The distribution graph shows no peaks at higher value than 175 nm which means nBA nanoparticles did not form agglomerates. If the integrity of the nBA particles had been compromised for any reason (for example, ultrasonic treatment during the solution preparation would have disrupted the lamellar structure or dispersing chemicals may disrupt the shape) there would be peaks in lower nm values in the distribution graph, but not lower value peak except peak of 175 nm is observed proves that nBA lamellar shape is intact. Undeformed lamellar shape of nBA is also seen in TEM micrograph (Fig. 3b).
Non-agglomerated lamellar nBA structure is observed in the micrograph of the TEM (Fig. 3b). A lonely lamellar nBA particle of approximately 70 × 200 nm is shown in the micrograph. In accordance with the conclusion of size distribution analysis, nBA particle stands alone in the prepared solution, it did not form agglomerates and the original lamellar structure is intact. Dispersing chemicals in the solution prevents agglomeration and preserve the original lamellar structure of nBA particles.
The nBA particles were not chemically modified to form a stable suspension in non-polar media (mineral base oil) in this study. The nBA particles were dispersed by means of suspension supporting chemicals and ultrasonic treatment. Although chemical modification is a widely used method to suspend nanoparticles, it is high cost method and may require very difficult chemical modification and purification steps [12, 22, 23]. In this study, we have achieved suspending nBA particles in mineral base oil with chemicals that have not been used for aim of dispersion or suspension of nanoparticles in non-polar media so far. The selected chemicals have amphiphilic properties. That is, these chemicals carry both hydrophobic and hydrophilic chemical functional groups in a single molecule. Such chemicals introduce hydrophobic and hydrophilic media to each other. Here hydrophilic part is nBA (log Kow value of boric acid is 0.18 which is stated as high polar) and hydrophobic part is mineral base oil. Amphiphilic molecules act as a mediator between two chemically dissimilar parts. Amphiphilic molecules interact and hold the boric acid with their hydrophilic side and adapts to the mineral base oil environment with its hydrophobic side. Thus, it makes the hydrophilic boric acid adaptable/dispersible in the hydrophobic environment and takes the role of mediator. These dispersing chemicals should be selected specifically for the molecules and environments to be introduced to each other [28]. Lutensol® XL 80 is a type of nonionic surfactant selected for its high ethoxy group content. Ethoxy group of this surfactant interacts with hydroxyl group of nBA. On the other hand, very long hydrocarbon side of Lutensol® XL 80, resembles the mineral base oil chemical structure and thus conforms to the engine oil environment. In addition, surfactants ensure finely-dispersed nano lubricants in non-polar mediums. By dispersing the lubricant particles, surfactants lead to form more uniform tribofilm between contacting surfaces [12]. High ethoxy content of Lutensol® XL 80 is very efficient to interact with boric acid hydroxyl groups; however, it has density value of about 1 g/cm3. Additives with high densities may sink in mineral base oil and suspension aim may fail. The additives themselves must remain suspended in the target media (as in the case of mineral base oil) or, when combined with other admixture chemicals, have the appropriate ratio in the appropriate chemical composition. Thus less dense amphiphilic chemical was used as nBA nanofluid solvent. Fatty alcohols are suitable for this purpose. They have long hydrocarbon chain with a hydroxyl group end of it. There are so few examples of liquid fatty alcohols with low density. Nonanol is an example, it has 0.8 g/cm3 density and it is liquid. Low density is suitable for mineral base oil medium, so it has not got tendency to sink at the bottom of mineral base oil container and it is chemically suitable for nBA to be suspended in mineral base oil successfully. Nonanol itself is not enough to suspend nBA in mineral base oil because of low polar group content, but it is compensated with Lutensol® XL80. The optimal chemical composition and ratio was determined.
3.2 Viscosity analysis of nBA nanofluid added mineral base oil
Viscosity values of original mineral base oil and nBA nanofluid added mineral base oil were examined as a function of time. Samples were heated to 25 °C, 40 °C and 100 °C then tested. In different percentages, nBA nanofluid was added to mineral base oil. All measurements were repeated three times, average values of these three measurements and the change rates in the viscosity of mineral base oil caused by nBA nanofluid additive were calculated in Table 1.
As seen in Table 1, adding nBA nanofluid to mineral base oil causes a decrease in original viscosity. The original viscosity of mineral base oil is 29.97 cP at room temperature, i.e. at 25 °C. Viscosity of original mineral base oil is decreased to 25.10 cP by adding 5% nBA nanofluid at 25 °C. The observed decrease in viscosity can be for two reasons. The first is the fact that solvent of nanofluid, nonanol, is less than mineral base oil (density of nonanol is 83 kg/m3, density of mineral base oil is 85 kg/m3). The viscosity of mineral base oil is expected to decrease with less dense nBA nanofluid addition. The second reason for decreasing the mineral base oil original viscosity may be the lamellar structure of nano-lamellar boric acid. This effect was also observed in the studies carried out with MoS2 and carbon nanotubes, which also have lamellar structures, by decreasing the viscosities of these base oils after addition of nanoparticles [29, 30].
The ratio of nBA nanofluid additive affects the percentage of decline of viscosity with direct proportion, that is, the change in viscosity increases when addition of nBA nanofluid to mineral base oil is in excess. This effect is due to the reasons mentioned above. Nonanol being less dense than mineral base oil and lamellar structure of nBA causes the original viscosity of mineral base oil to decrease. This should be taken into account when determining the appropriate amount of addition of nBA nanofluid into mineral base oil. With the help of other characterizations, an optimal addition percentage will be determined. Therefore, this information is obtained from the viscosity characterization that addition percentage of nBA nanofluid into mineral base oil should be 1 or 3, that is, not 5, because adding 5% of nBA nanofluid affect the original viscosity of mineral base oil much more than 1 and 3 percentages.
Although the viscosity of nBA nanofluid added mineral base oil decreases in the general, it is observed that this effect decreases as the temperature increases. In Fig. 4, it is clearly seen that the viscosity value of nBA nanofluid added mineral base oil samples approach the original mineral base oil viscosity value at high temperatures. Two samples with different ratios of nBA nanofluid addition appears to be very close to the original mineral base oil viscosity value at 100 °C. At 100 °C, 1% of nBA nanofluid addition to the oil increases the original viscosity by 1% and 3% addition decreases the original viscosity by 2.88%. Mineral base oil with 5% nBA nanofluid has a viscosity value far from original viscosity of mineral base oil even at high temperatures. In this evaluation, which will be concluded with the evaluation of other characterizations, the 5% nBA nanofluid contribution rate will remain a non-favorable option.
3.3 nBA nanofluid effect on mineral base oil friction coefficient
Tribometer test was performed to observe the effect of nBA on the friction coefficient of the mineral base oil. Boric acid has superior characteristics that have lamellar structure. In order to achieve lubrication between sliding surfaces, it can be an option to add nBA to engine oils. Tribometer test alone is not sufficient to propose a material as a motor oil additive. The tribometer test with the ball-on-plate method was performed as a basic test for the characterization of the nBA nanofluid lubrication feature and to provide a prediction. In this study, in which we focused on the suspension of the nBA nanoparticle in a non-polar environment, a tribometer test was performed with the ball-on-plate method to examine the effect of the nBA particle on the friction coefficient of mineral base oil.
Table 2 shows values of friction coefficients of non-added mineral base oil and mineral base oil with different ratios of nBA nanofluid. The table also presents calculated percent reduction on friction coefficient values by nBA addition.
It is seen that adding nBA nanofluid does not affect negatively the friction coefficient of mineral base oil. All three ratios of nBA nanofluid addition was tested to examine the effect of nBA on friction. Nonanol is less dense than mineral base oil; consequently, adding nBA nanofluid (include nonanol as solvent) to mineral base oil decreases the viscosity and this may result increasing the friction coefficient. Lowering viscosity increases the friction coefficient; however, with the help of the nBA lubricant nBA nanofluid compensate the negative effect of decreasing viscosity and by 3% nBA nanofluid addition, the COF value of original mineral base oil decreases by 3%. In the case of 5% addition of nBA nanofluid to mineral base oil, nonanol percentage is also increased and viscosity also decreased much more than 3% addition. In case the viscosity decreases caused by nonanol and the friction coefficient increases accordingly, it is seen that the 5% addition of nBA nanofluid decreases the friction coefficient (2.54%) less than 3% (2,91%). In the case of 5% addition of nBA nanofluid to mineral base oil, the effect of increasing the COF value by nonanol has been observed to prevent the lubrication property of the nBA nanoparticle eventually. Thus, it was concluded that in the light of viscosity and tribometer characterizations the optimal ratio of nBA nanofluid addition into mineral base oil was 3%. Suspension characterization will be made according to this addition ratio.
Figure 5 shows graphs of friction coefficient values of mineral base oil and nBA nanofluid added mineral base oil with respect to sliding distance. Two graphs are superimposed to examine the difference clearly. Blue line belongs to non-added mineral base oil, red line belongs to 3% nBA nanofluid added mineral base oil and red line appears under blue line. Lower line shows that nBA nanofluid decreases the friction coefficient of mineral base oil. The nBA particles were in nano lamellar structure as declared in Fig. 1, which provides a superior sliding property. When nBA platelets were squeezed into two sliding surfaces, the layers slide over each other to help reducing the friction of two surfaces. Friction tests also revealed this feature.
3.4 Suspension characterization of nBA nanofluid added mineral base oil
In the light of viscosity and tribometer measurements, the most effective and optimum nBA nanofluid ratio was determined as 3%. Suspension of this additive ratio was characterized by turbidimetry characterization technique. Conducting suspension characterizations of added mineral base oil by Turbiscan Tower® is a novel aspect of this study. This technique, in short, measures the mobility of ingredients within the sample with changes in time-dependent Transmittance (T) and Back Scattering (BS) values. Turbiscan graphs are drawn with T and BS intensities with respect to height of the sample holder. Different intensities on different height of the sample holder are logged. The displacement of the nanoparticles within the sample is indicative of instability in long term. According to Derjaguin, Verway, Landau, and Overbeek (DVLO) theory the suspension is not stable if two particles collides [20]. As the movement of particles increases, collision probability increases, and DLVO theory claims the fact that if particles collide, suspension is not stable.
The particle mobility in the sample is documented by recording the T and BS intensities of the sample over time with the turbiscan instrument. Different values in these parameters over time; i.e., the movement of the nanoparticles in the sample and the displacement of the particles, leads to high fluctuation in sedimentation profile. Therefore, they have high Turbiscan Stability Index (TSI) values. TSI parameter indicates the numeric values of suspension stability by taking account the information of both T and BS graphs. The suspension of a solution with high TSI values is not stable. Low TSI values and low fluctuations in the sedimentation profiles are interpreted as high stability. The color scale indicator on the right shown in Fig. 6 is time. Turbiscan instrument records the sample’s placement in the turbiscan instrument for characterization, as zero, and receives its first scan. Then, as time passes, it refreshes the scan at certain time intervals and shows each scan with a different color. The time-dependent intensity values of mineral base oil containing nBA nanofluid are monitored by this color scale. In Fig. 6, all these colors are overlapped, as there is almost no change in T and BS parameter with time. This shows high stability of the suspension. Numeric values of these graphs were calculated by software program of Turbiscan Tower® that is Formulaction® and presented in Table 3. nBA nanofluid added mineral base oil presents very narrow fluctuation (Fig. 6) hence very low TSI value (Table 3) that interpreted as stable suspension.
One of the most important aspects of using the turbiscan instrument is that a suspension test can be expressed in scientific and numerical value. In this study, while conducting the suspension studies of nBA particles, we attached great importance to expressing our success with scientific and measurable values. Another important aspect is, in a sense, to advance time. Turbiscan® instrument detect any small changes 50 times faster than naked eye [31]. This means that a short-term experiment on this instrument can show the visible instability of the suspension of much longer time. The high stability of nBA with low TSI values, which we observed in the laboratory for more than 3 months, confirms this conclusion. The high stability interpretation about nBA nanofluid added mineral base oil by the 24-h test at Turbiscan is also seen in real-time bench tests performed for a period of 3-months. nBA particles remain successfully suspended in mineral base oil for 3 months.
In studies about boric acid suspension, researchers use very difficult and costly methods to keep boric acid suspended and they do not state the stability duration times. For example, Kim et al. [12] uses ball milling for 10 h to reduce the size of boric acid particles and to suspend them in mineral base oil. By using the nano-structured lamellar boric acid and using the suspension method developed in this study, we were able to reduce this preparation time to less than 1 h. In addition, high suspension duration of our samples (more than 3 months) compared to other nanoparticle suspension studies indicates that these results represent the longest times to keep a nanoparticle suspended in a non-polar media [8,9,10,11, 32].
4 Conclusion
In the present work, nano structured lamellar boric acid (nBA) was suspended in mineral base oil (base oil, a model for non-polar media). For this purpose, a new procedure was developed for a nanoparticle to be suspended in a nonpolar environment with the help of unique suspension enhancing chemicals. The main findings and conclusions of this study can be summarized as follows:
-
A simple and novel method is developed to suspend nBA in non-polar media, by using a combination of ultrasonic treatment and two special suspension chemicals (Lutensol® XL 80 and nonanol) that are not previously used for this purpose.
-
Nonanol is less dense than mineral base oil; therefore, it decreases the viscosity of original mineral base oil. As temperature increases, effect of nonanol in nBA nanofluid was became minor and lubrication characteristics of nBA reveals. At 100 °C, 3% nBA nanofluid addition to mineral base oil decreases the original mineral base oil viscosity value by less than 3%.
-
nBA nanofluid does not affect the friction coefficient of mineral base oil negatively. Friction coefficient values of nBA nanofluid added mineral base oil samples were measured by UTS Tribometer T30M-HT® with a reciprocating test module. Measured friction coefficient lowering is 3% at 100 °C for 3% addition ratio of nBA nanofluid into mineral base oil.
-
Suspension is characterized by turbidimetry method by means of Turbiscan® instrument. This method gives scientific and numeric value of suspension stability. Turbiscan Stability Index (TSI) of mineral base oil with 3% added nBA nanofluid implies that the suspension will remain stable for months (because Turbiscan® instrument detect any small changes faster than naked eye [31]), and our observations in the laboratory conditions confirm that no sedimentation has been observed for more than 3 months.
References
Yu W, Xie H (2012) A review on nanofluids: preparation, stability mechanisms, and applications. J Nanomater 2012:17
Ghadimi A, Saidur R, Metselaar HSC (2011) A review of nanofluid stability properties and characterization in stationary conditions. Int J Heat Mass Transf 54(17):4051–4068
Demas NG et al (2017) Experimental evaluation of oxide nanoparticles as friction and wear improvement additives in motor oil. J Nanomater 2017:12
Jendrzej S, Gökce B, Barcikowski S (2017) Colloidal stability of metal nanoparticles in engine oil under thermal and mechanical load. Chem Eng Technol 40(9):1569
Kolodziejczyk L et al (2007) Surface-modified Pd nanoparticles as a superior additive for lubrication. J Nanoparticle Res 9(4):639–645
Mahbubul IM et al (2015) Effective ultrasonication process for better colloidal dispersion of nanofluid. Ultrason Sonochem 26:361–369
Chatrchyan S, Khachatryan V, Sirunyan AM et al (2014) Measurement of pseudorapidity distributions of charged particles in proton–proton collisions at √=8 TeV by the CMS and TOTEM experiments. Eur Phys J C 74:3053. https://doi.org/10.1140/epjc/s10052-014-3053-6
Laad M, Jatti VKS (2018) Titanium oxide nanoparticles as additives in engine oil. J King Saud Univ Eng Sci 30(2):116–122
Yu Oganesova E, Lyadov A, Parenago OP (2018) Nanosized additives to lubricating materials. Russ J Appl Chem 91:1559–1573
Sui T et al (2015) Effects of functional groups on the tribological properties of hairy silica nanoparticles as an additive to polyalphaolefin. RSC Adv 6:393–402
Amiruddin H (2015) Stability of nano-oil by pH control in stationary conditions. Procof Mech Eng Res Day 2015:55–56
Erdemir A (2005) Review of engineered tribological interfaces for improved boundary lubrication. Tribol Int 38(3):249–256
Kim JH et al (2012) Effect of surfactant on tribological performance and tribochemistry of boric acid based colloidal lubricants. Tribol Mater Surf Interfaces 6(3):134–141
Koshy CP, Rajendrakumar PK, Thottackkad MV (2015) Evaluation of the tribological and thermo-physical properties of coconut oil added with MoS2 nanoparticles at elevated temperatures. Wear 330–331:288–308
Rao KP, Xie CL (2006) A comparative study on the performance of boric acid with several conventional lubricants in metal forming processes. Tribol Int 39(7):663–668
Erdemir A, Fenske GR, Erck RA (1990) A study of the formation and self-lubrication mechanisms of boric acid films on boric oxide coatings. Surf Coat Technol 43–44:588–596
Shah FU, Glavatskih S, Antzutkin ON (2013) Boron in tribology: from borates to ionic liquids. Tribol Lett 51(3):281–301
Erdemir A (1991) Tribological properties of boric acid and boric-acid-forming surfaces. Part I. Crystal chemistry and mechanism of self-lubrication of boric acid. Lubr Eng 47:72–168
Lovell MR et al (2010) Influence of boric acid additive size on green lubricant performance. Philos Trans R Soc A Math Phys Eng Sci 368(1929):4851–4868
Godet M (1984) The third-body approach: a mechanical view of wear. Wear 100(1):437–452
Gulzar M et al (2016) Tribological performance of nanoparticles as lubricating oil additives. J Nanoparticle Res 18(8):223
Rs P, Krishna V, Gurram K (2015) Experimental evaluation of nano-molybdenum disulphide and nano-boric acid suspensions in vegetable oils as prospective cutting fluids during turning of AISI 1040 steel. Proc Inst Mech Eng Part J J Eng Tribol 230:208–210
Wiśniewska M, et al (2013) Effect of polyacrylic acid (PAA) adsorption on stability of mixed alumina–silica oxide suspension. Powder Technol 233:190–200
Wiśniewska M et al (2013) Stability of colloidal silica modified by macromolecular polyacrylic acid (PAA)–application of turbidymetry method. J Macromol Sci Part A 50(6):639–643
Santos J, Calero N, Muñoz J, (2016) Optimization of a green emulsion stability by tuning homogenization rate. RSC Adv 6(62):57563–57568
Álvarez Cerimedo MS et al (2010) Stability of emulsions formulated with high concentrations of sodium caseinate and trehalose. Food Res Int 43(5):1482–1493
Qi X et al (2017) Application of Turbiscan in the homoaggregation and heteroaggregation of copper nanoparticles. Colloids Surf A Physicochem Eng Asp 535:96–104
Mengual O et al (1999) Characterisation of instability of concentrated dispersions by a new optical analyser: the TURBISCAN MA 1000. Colloids Surf A Physicochem Eng Asp 152(1):111–123
Wan Q et al (2014) Rheological and tribological behaviour of lubricating oils containing platelet MoS2 nanoparticles. J Nanoparticle Res 16(5):2386
Chen L et al (2008) Nanofluids containing carbon nanotubes treated by mechanochemical reaction. Thermochim Acta 477(1):21–24
Yadav T, Mungray AA, Mungray AK (2015) A comparative analysis of a TiO2 nanoparticle dispersion in various biological extracts. RSC Adv 5(79):64421–64432
Sui T et al (2015) Effect of particle size and ligand on the tribological properties of amino functionalized hairy silica nanoparticles as an additive to polyalphaolefin. J Nanomater 2015:9
Acknowledgement
AG thanks Turkish Academy of Sciences (TUBA) for partial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tanrıseven, Z., Gül, A. & Özayman, M. A simple route to suspend boric acid in non-polar media. SN Appl. Sci. 2, 1604 (2020). https://doi.org/10.1007/s42452-020-03385-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03385-8