Abstract
We implemented molecular dynamics simulations to study the effect of single-walled carbon nanotube (SWCNT) and carboxylic (–COOH) functionalized SWCNT on the crosslinking and interfacial behavior of Epon 862 nanocomposite. Results showed that the introduction of control SWCNT in the Epon system reduced the crosslinking by 8–12% in comparison to control system (without CNT). The molecules of Epon 862 and Epikure-W changed their conformation and aligned themselves in the direction parallel to the surface wrapping the nanotubes. Also, no interfacial bonding was found between the polymer and SWCNT. However, the introduction of the –COOH functional groups on the surface of SWCNT lead to increase in crosslinking and resulted in a strong bond formation between the polymer and nanotube interphase. A significant increase in energy was found in Epon/COOH-SWCNT systems that lead to an increase of 33% in interfacial strength in comparison to Epon/SWCNT counterpart. The pullout simulations of Epon/SWCNT samples showed separation at the interphase with no polymer atoms attached to CNT. In contrast, bond stretching and breakage were observed in the polymer chain, whereas, a strong interphase was observed in Epon/COOH-SWCNT samples. This study reveals the nanolevel interactions between the nanotubes and polymers which otherwise not possible through experimental techniques, and lead to cognize that the functionalization of SWCNT with –COOH groups can establish a strong network with the epoxy chain and significantly enhance the interfacial properties particularly for applications where the interphase is critical.
Similar content being viewed by others
1 Introduction
Prediction of mechanical properties and modeling the mechanical behavior of the CNT reinforced polymeric composites (RPCs) has attracted great attention [1,2,3] especially considering their unique structural and electronic properties [4, 5]. High strength, large aspect ratio and low density of CNTs have made these particles an ideal reinforcement candidate in polymeric composites for next generation applications such as in aerospace, packaging, automotive and civil industries. However, a number of experimental investigations in the literature reported contradicting crosslink density and macroscopic mechanical properties considerably lower than the theoretically predicted values [6,7,8]. It is well known that the macroscopic properties of CNT RPCs are directly depends on the extent of crosslinking between the epoxy resin and its curing agent, CNT dispersion and its interfacial bonding with the epoxy system. Also, the interfacial strength, a key property responsible for maximizing the load transfer has been investigated using the fiber pull-out or push-out tests [9, 10]. Fiber pull-out from a matrix is usually characterized by the critical shear stress to debond the CNT/polymer interface and the subsequent interface friction of the CNT and polymer. Results demonstrated that the magnitude of interfacial bonding is limited and thus the load transfer ability in CNT RPCs is weak because of the atomically smooth, non-reactive nature of the nanotube outer surface [11, 12]. The load transfer between CNTs and polymer matrix takes primarily due to weak Van der Waals forces. However, the authors and other researchers showed recently through macroscopic experimental teiques that a strong covalent bonding between the CNTs and polymer was formed by making the outer surface of CNTs reactive through chemical functionalization with carboxylic (COOH) or amino (NH2) groups [13,14,15,16,17]. As a result, the mechanical and thermal properties enhanced significantly due to improved interfacial bonding and better crosslinking between the CNTs and polymer. However, no microscopic level experimental testing proving the improvement in interfacial bonding between the functionalized CNT and matrix such as fiber pull-out are conducted, whereas, only a few studies are reported on non-functionalized CNT RPCs [18, 19].
In addition, the crosslinking of epoxy molecules and its interaction with the CNT is a nanolevel phenomenon. The interface region between the CNT and epoxy molecules is reported to be less than 5 nm. Due to the complexity and lack of microlevel experimental techniques, researchers over the years have extensively focused on theoretical methods for predicting the properties of CNT RPCs. It is recognized that atomic simulations based on high-fidelity forcefields models can provide a better understanding of interfacial bonding and load transfer mechanism between the CNTs and the polymer matrix [20, 21]. Currently, Molecular dynamics (MD) simulation technique has been widely used for studying these properties due to its accuracy, flexibility, and various force fields [1, 22,23,24,25]. Liao and Li [26] evaluated the interface of a CNT reinforced polystyrene composites. However, no chemical bonding was considered in their simulation, and thus the interfacial adhesion primarily resulted from weak Van der Waals interaction and mismatch in the coefficient of thermal expansion.
On the other hand, several researchers studied the influence of chemical crosslinks between a single-walled carbon nanotube (SWCNT) and a polymer matrix and reported that forming few crosslinks between CNT and polymer, the crosslinking and interfacial strength increased significantly. However, the simulations in these studies were performed on uncured epoxy models. In recent years, MD simulations to predict interfacial bonding/stresses were extended further by considering the cured epoxy model with and without the SWCNT reinforcement which is more realistic than uncured CNT/polymer model [26,27,28]. However, again, no chemical crosslinking was considered between nanotubes and polymer in these studies and the pullout simulations were based on weak Van der Waals interaction.
Although to the best of our knowledge, a considerable MD simulation study is done to understand the interfacial bonding between the CNT and polymer matrix, many of these investigations were performed by considering the weak Van der Waals interactions and by considering the uncured/cured polymer models. Thus, a huge variation in interfacial strength of CNT RPCs is reported in these studies [1, 22,23,24,25,26,27,28]. The variation in interfacial strength may be acceptable due to the fact that the simulations were performed on various polymers and with different model set-up. However, the predictions based on weak Van der Waals forces are questionable, specifically when polymer is modified with functionalized nanotubes. Modification of CNTs induces strong covalent bonds with matrix [13,14,15,16,17], thus predicting the interfacial bonding based on the Van der Waals interaction will not yield dependable results.
Therefore, in this work, a comprehensive fundamental MD simulations was performed to understand the variation in crosslinking behavior with the introduction of SWCNT and carboxylic (–COOH) functionalized SWCNT, and compared with the neat Epon composites. Also, the interfacial strength of non-functionalized and –COOH functionalized RPCs was investigated by performing the CNT pull-out simulations.
2 Simulation method: crosslinking procedure
Large-scale atomic/molecular massively parallel simulator (LAMMPS) was used to carry MD simulations. The simulations were performed mimicking the RPCs processing procedure suggested by the manufacturer using a mixing ratio of Epon 862 (resin) and Epikure-W (curing agent) as 100:26.4. Initially, non-crosslinked Epon 862 and Epikure-W molecules were packed into an amorphous cell. Initial parameters like density of 1.17 gm/cc3 and a temperature of 393 K was applied. The crosslinking simulations were then performed using Dreiding forcefield and LAMMPS software. Systems of 100 molecules of Epon 862 and 50 molecules of Epikure-W were used to create structures. The diameter and length of carbon nanotubes were 6.78 Å and 27 Å respectively.
The molecular model of cured Epon resin was developed using MAPS software following the same procedure reported by Varshney et al. [28]. Initial system was equilibrated using NPT simulations at 300 K and atmospheric pressure for 100 ps. The temperature was controlled using Nose–Hoover thermostat and barostat, and the possibility of any surface effects was minimized by applying 3D periodic boundary conditions. The crosslinking was performed after equilibrating the system using several steps which includes optimization, relaxation and then multistep algorithm which created new bonds after each cycle. Cyclic process of cross-linking continued until a specified target was achieved or there was no more possibility of crosslinking left or the reactive sites moved far away. Figure 1 shows the system model system and activation steps of reactive sites. Consequently, the final cured model of neat Epon system is shown in Fig. 2.
The cured model of Epon/SWCNT was developed using the similar crosslinking procedure followed for the neat Epon system shown in Fig. 3. The Epon 862, Epikure-W and SWCNT were allowed to react in amorphous cell. However, for the Epon/COOH-SWCNT system, at first, one-side of SWCNT surface was functionalized by manually placing the –COOH groups at random position. The functional groups were then activated and placed in an amorphous cell with activated Epon 862 and Epikure-W molecules. The surface of the SWCNT was modified with –COOH groups which tend to form strong covalent bonding with the epoxy groups of the Epon 862 system shown in Fig. 4 . During the curing reaction, hydrogen atoms in the amine (NH2) groups of a cured epoxy molecule reacts with the COOH groups of SWCNT as shown in Fig. 4b, c. Finally, multistep algorithm was performed to create bonds between the activated reaction sites of Epon 862, Epikure-W and COOH-SWCNT. The final cured model of Epon/COOH-SWCNT system showing bonds formation at the interface is represented in Fig. 5.
3 Interfacial properties
The interfacial bonding energy of Epon/SWCNT and Epon/COOH-SWCNT systems were determined using pullout simulations by applying a displacement controlled load on the CNT. Interaction energy of the system was estimated from the energy difference, i.e. between the total energy of system and sum of energies of Epon and SWCNT system using Eq. 1.
where \( E_{Total} \) is the total energy of Epon/SWCNT system, \( E_{nanotube} \) is the energy of CNT and \( E_{polymer} \) is the energy of polymer.
In this study, the SWCNT was set free, while the polymer and amorphous cell were fixed. Energy required to completely pullout the CNT from the system was calculated using Eq. 2.
where E1 and E2 are the initial and final energy during pullout.
Finally, the interfacial shear stress (Ʈi) was determined using Eq. 3.
where r and L are the radius and length of nanotube.
Steered molecular dynamics (SMD) simulations were employed to simulate the pullout of the SWCNT from the Epon 862 system. The potential mean force was calculated from non-equilibrium process such as SMD. By applying a moving spring force to the center of mass of the SWCNT’s atoms, the SWCNT was pulled along z-axis at a constant speed, while all other sides were constrained.
4 Results and discussion
4.1 Epon system crosslinking
Crosslinking algorithms were continued upto10 iterations for a time length of 1 ns. The amount of crosslinking was recorded with every iteration. Once the crosslinking process of neat Epon system started, the simulations continued until all the maximum possible activated reactive sites were crosslinked or the system completed the set 10 iterations. At this point, to confirm the accuracy of the crosslinked neat Epon system, the glass transition temperature (Tg) was predicted with the annealing method and compared with the experimental, simulated and manufacturer reported Tg. The temperature of the system was raised from 300 K to 520 K and the Tg was determined from the density versus temperature response shown in Fig. 6. Result showed a Tg of 397 k or 122.85 °C calculated using the discontinuity in the graph is in good agreement with the reported value of 120–135 °C [29, 30].
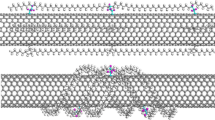
After assuring the accuracy of the neat system, identical crosslinking algorithm were continued for Epon/SWCNT system, and the intial and final crosslinking densities were measured. The initial crosslinking density of this system after first iteration was reduced by 5% in comparison to neat Epon system. Also, the Epon/SWCNT system reached its maximum crosslink density of 72–75% within the set number of iterations which is 11% lower in comparison to neat counterpart shown in Fig. 7. The decrease in crosslink density of Epon/SWCNT can be attributed to the presence of SWCNT itself, which is not only non-reactive with the Epon system, but also found to block and move away the reactive sites of Epon 862 and Epikure-W molecules as shown in Fig. 3b. Another interesting phenomenon observed in our simulation is the polymer wrapping around SWCNT shown in Fig. 8. The aromatic rings of Epon 862 and Epikure W were found to gradually align parallel and wrap the nanotube surface. Also, due to the absence of functional groups on SWCNT, no interfacial bonding was observed with Epon862 system’s reactive sites as shown in Fig. 3b. Eventhough, the desired higher crosslink density and interfacial bonding between the SWCNT and Epon system was not found in this study, the improvements in mechanical properties reported by other researchers through experiments and simulations could be due to the polymer wrapping of CNTs surface and strong inherent tensile strength and elastic modulus of CNT itself. The polymer wrapping around the CNT surface is helpful in preventing them from aggregating into bundles and improving their dispersion in a epoxy composite.
On the otherhand, the initial and maximum crosslink density of neat Epon and Epon/SWCNT-COOH systems were found higher than the Epon/SWCNT conterpart. The higher crosslinking density in the neat system could be attributed to the availability of more Epon and Epikure-W reactive sites that were blocked or moved away by the presence of SWCNT in the Epon/SWCNT system. In contrast, for the Epon/COOH-SWCNT system, eventhough the reactive sites were blocked similar to Epon/SWCNT system, the presence of –COOH functional groups on CNT surface established a strong bonding with the reactive sites of polymer as shown in Fig. 5 and illustrated in Fig. 4, respectively.
4.2 Interfacial properties
Snapshots of SWCNT and SWCNT-COOH being pulled from the Epon system are shown in Figs. 9 and 10. It can be seen from Fig. 9, that the surface of SWCNT after pullout was very smooth with no polymer attached to it indicating no interfacial bonding. Therefore, the only interaction between the SWCNT and Epon system expected is may be due to the weak Van der Waals forces considered by other researchers [22,23,24, 26,27,28] However, in Epon/COOH-SWCNT system, when higher pull-out force were applied, no separation was observed at the interface between SWCNT and polymer. Instead, the friction/shearing was initiated in the polymer molecules, and lead to complete breakage of polymer chain bonds, while a strong interphase was found between between the Epon/COOH-SWCNT evident from Fig. 10. Similar higher pull-out forces for a capped CNT than that of the corresponding open-ended CNT was reported by Li et al. implilying a significant contribution from the CNT cap to the interfacial properties [31].
The energy trend in the systems monitored during the pull-out is shown in Fig. 11. The initial and final energy in Epon/SWNCT and Epon/COOH-SWCNT measured during the pullout was 13,500 kcal/mol and 12,000 kcal/mol, and 15,200 kcal/mol and 13,200 kcal/mol, respectively. A significantly higher initial and final energy was found in the Epon/COOH-SWNCT system in comparison to Epon/SWCNT counterpart. Similarly, the interfacial shear strength in these system was found 97.84 MPa and 130.46 MPa, with a increase of 33% in the Epon/COOH-SWNCT system. These significant enhancements in the system energy and interfacial shear strength in Epon systems reinforced with –COOH functionalized SWCNT could be attributed to the formation of a strong covalent bond at the interface. It is to be noted that in this study, the carboxylic functional groups were placed on one side of CNT surface. We expect the enhancement of these properties in the nanotube RPCs to be even significantly higher than neat composites.
5 Conclusion
In this study, MD simulations was successfully utilized to predict the crosslinking behavior and interfacial properties of Epon 862 systems reinforced with non-functionalized and –COOH functionalized SWCNT.
The crosslinking density reduced when SWCNT was introduced into the neat Epon system as a result of reactive site blockage and no interface bonding between nanotubes and polymer. Another interesting phenomenon observed was polymer wrapping, which could potentially reduce the re-agglomeration of CNTs in the epoxy system, depending on the uniformity of dispersion, percentage of CNT concentration, and the carbon–carbon attraction energy. Hence, the enhancements in mechanical properties reported by the researchers in epoxy systems at lower concentrations of non-functionalized CNTs could be due to the inherent properties of CNTs combined with polymer wrapping and weak Van der Waals forces at the interphase.
In contrast, the surface functionalization of SWCNT with –COOH groups established a strong interfacial bonding with the Epon system that lead to significant enhancement in crosslink density and interfacial strength. Pull out simulations showed polymer chain sliding, shearing and finally leading to breakage instead of separation at the interphase in Epon/COOH-SWCNT systems. This results indicated that by functionalizing nanotubes with combatible functional groups such as carboxylic, amine etc., a highly crosslinked system with stronger interface can be developed which could be potent than the crosslinking of base polymer molecules chain itself.
References
Gou J, Minaie B, Wang B, Liang Z, Zhang C (2004) Computational and experimental study of interfacial bonding of single-walled nanotube reinforced composites. Comput Mater Sci 31:225–236
Thostenson ET, Chou T (2002) Aligned multi-walled carbon nanotube-reinforced composites: processing and mechanical characterization. J Phys D 35:77–80
Copper CA, Cohen SR, Barber AH, Wagner HD (2002) Detachment of nanotubes from a polymer matrix. Appl Phys Lett 81:3873–3875
Lambin P, Fonseca A, Vigneron JP, Nagy JB, Lucas AA (1995) Structural and electronic properties of bent carbon nanotubes. Chem Phys Lett 245:85–89
Mintmire JW, White CT (1996) Electronic structure simulation of carbon nanotubes. Synth Met 77:231–234
Varshney V, Patnaik SS, Roy AK, Farmer BL (2008) A molecular dynamics study of epoxy-based networks: cross-linking procedure and prediction of molecular and material properties. Macromolecules 41:6837–6842
Mamedov AA, Kotov NA, Prato M, Guldi DM, Wicksted JP, Hirsch A (2002) Molecular design of strong single-wall carbon nanotube/polyelectrolyte multilayer composites. Nat Mater 1:190
Frankland SJV, Calgar A, Brenner DW, Griebel M (2002) Molecular simulation of the influence of chemical cross-links on the shear strength of carbon nanotube-polymer interfaces. J Phys Chem B 106:3046–3048
Piggott MR (1995) A new model for interface failure in fiber reinforced polymers. Compos Sci Technol 55(3):269–276
Tsuda T, Ogasawara T, Deng F, Takeda N (2011) Direct measurments of interfacial shear strength of multi-walled carbon nanotube/PEEK composite using a nano-pullout method. Compos Sci Technol 71:1295–1300
Zhu J, Kim J, Peng H, Margrave JL, Khabashesku VN, Barrera EV (2003) Improving the dispersion and integration of single-walled carbon nanotubes in epoxy composites through functionalization. Nano Lett 3(8):1107
Qian D, Dickey EC, Andrews R, Rantell T (2000) Load transfer and deformation mechanisms in carbon nanotube-polystyrene composites. Appl Phys Lett 76(20):2868–2870
Rahman MM, Zainuddin S, Hosur MV, Robertson CJ, Kumar A, Trovillion J, Jeelani S (2013) Effect of NH2-MWCNTs on crosslink density of epoxy matrix and ILSS properties of e-glass/epoxy composites. Compos Struct 95:213–221
Salam MB, Hosur MV, Zainuddin S, Jeelani S (2013) Improvement in mechanical and thermo-mechanical properties of epoxy composite using two different functionalized multi-walled carbon nanotubes. Open J Compos Mater 3:1–9
Jahan N, Narteh AT, Hosur M, Rahman M, Jeelani S (2013) Effect of carboxyl functionalized MWCNTs on the cure behavior of epoxy resin. Open J Compos Mater 3:40–47
Bahr JL, Tour JM (2002) Covalent chemistry of single-wall carbon nanotubes. J Mater Chem 12(7):1952–1958
Sinnott SB (2002) Chemical functionalization of carbon nanotubes. J Nanosci Nanotechnol 2(2):113–123
Barber AH, Cohen SR, Wagner HD (2003) Measurement of carbon nanotube-polymer interfacial strength. Appl Phys Lett 82(23):4140–4142
Dutta AK, Penumadu D, Files B (2004) Nanoindentation testing for evaluating modulus and hardness of single-walled carbon nanotube reinforced epoxy composites. J Mater Res 19:158
Grujicic M, Cao G, Roy WN (2004) Atomistic modeling of solubilization of carbon nanotubes by non-covalent functionalization with poly (p-phenylenevinylene-co-2,5-dioctoxy-phenylenevinylene. Appl Surf Sci 227:349–363
Grujicic M, Cao G, Roy WN (2004) Atomistic simulations of the solubilization of single-walled carbon nanotubes in toluene. J Mater Sci 39:2315–2325
Zaminpayma E, Mirabbaszadeh K (2012) Interaction between single-walled carbon nanotubes and polymers: a molecular dynamics simulation study with reactive force field. Comput Mater Sci 58:7–11. https://doi.org/10.1016/j.commatsci.2012.01.023
Odegard GM, Clancy TC, Gates TS (2005) Prediction of mechanical properties of polymers with various force fields. In: Structures, structural dynamics, and materials and co-located conferences. pp 18–21. http://doi.org/10.2514/6.2005-1850
Hartmann S, Wunderle B, Hölck O (2012) Pull-out testing of SWCNTs simulated by molecular dynamics. Int J Theor Appl Nanotechnol. https://doi.org/10.11159/ijtan.2012.009
Brenner DW, Shenderova OA, Harrison JA, Stuart SJ, Ni B, Sinnott SB (2002) A second-generation reactive empirical bond order (REBO) potential energy expression for hydrocarbons. J Phys: Condens Matter 14:783–802
Liao K, Li S (2001) Interfacial characteristics of a carbon nanotube-polystyrene composite system. Appl Phys Lett 79(25):4225–4227
Lordi V, Yao N (2000) Molecular mechanics of binding in carbon-nanotube polymer composites system. J Mater Res 15(12):2770–2779
Frankland SJV, Harik VM (2003) Analysis of carbon nanotube pull-out from a polymer matrix. Surf Sci 525(1–3):103–108
Li C, Strachan A (2010) Molecular simulations of crosslinking process of thermosetting polymers. Polymer 51(25):6058–6070. https://doi.org/10.1016/j.polymer.2010.10.033
Theodore M, Hosur M, Thomas J, Jeelani S (2011) Influence of functionalization on properties of MWCNT–epoxy nanocomposites. Mater Sci Eng A 528:1192–1200
Li Y, Liu S, Hu N, Han X, Zhou L, Ning H, Wu L, Alamusi, Yamamoto G, Chang C, Hashida T, Atobe S, Fukunaga H (2013) Pull-out simulations of a capped carbon nanotube in carbon nanotube-reinforced nanocomposites. J Appl Phys 113:P144304
Acknowledgements
The authors like to thank the National Science Foundation (NSF) for supporting this work through Grants: NSF HRD1409918, NSF HRD 18186896 and NSF DMR-1659506.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MP4 39827 kb)
Rights and permissions
About this article
Cite this article
Syed, F., Zainuddin, S., Willis, A. et al. Crosslinking and interfacial behavior of carboxylic functionalized carbon nanotube Epon nanocomposites: a molecular dynamic simulation approach. SN Appl. Sci. 1, 1423 (2019). https://doi.org/10.1007/s42452-019-1441-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-1441-0