Abstract
A new Schiff base (L) was synthesized and confirmed by 1HNMR, FTIR spectroscopy. The modified PVC with new Schiff base (L) was synthesized to produce a homogenous blend (PVC-L). A homogenous blend (PVC-L) added to copper chloride to produce PVC-L-Cu(II). The PVC films had been irradiated with an ultraviolet light for long period and confirmed by FTIR spectroscopy, weight loss and surface morphology was investigated by scanning electron microscopy.
Similar content being viewed by others
1 Introduction
The surface is modified by surface grafting method, without changing in the bulk properties. Mostly, the effect found for surface parameters may be detected both by the bulk polymer radical chemical modifications and modification influenced accurately at surface level, without modifying the structure and the bulk polymer properties. Surface graft polymerization used to modify the surfaces of membrane, using various surface activation methods included UV-irradiation [1], treatment of plasma [2], ozone [3], and chemical initiator [4] which can performed by either living graft polymerization or free radical graft polymerization. The direct modification onto the surface of polymer produces pore sizes change and may result to develop the polymeric membrane performance [5]. Graft copolymerization includes the reaction of already formed homopolymer or copolymer with fresh monomers [6] which are covalently bonded within polymeric chains, and thus conventional polymerization ways can be used. These techniques involve chemical, radiation, photochemical, and plasma-induced ways [7, 8]. Graft polymerization is also used in PVC modification to improve the performance of the polymer derivate and to exploit their application area [5]. The aim of this work, modification of PVC and a Schiff base containing heterocyclic aromatic ring with Copper(II), after irradiation using UV for a long period. In addition, the Schiff base improved PVC photostability upon photodegradation. Weight loss and surface morphology of PVC films were affected by transition metal. These heterocyclic aromatic units are connected to PVC chains as pending groups will greatly improve PVC photostability.
2 Materials and methods
2.1 Instrumentation
A record of FTIR spectra was conducted using Jasco FTIR-4200 spectrometer (Tokyo, japan) (KBr disc). QUV Acceleration weather-meter tester (by Q-Band company, USA) was deployed to study the morphology changes of the PVC films prepared samples. This morphology investigation was performed utilizing Inspect S50 microscope (by FEI company, Czech Republic) while EDX patterns were examined by Bruker XFlash 6/10 (Japan).
3 Materials
The entire chemicals used in this work were of highest purity available and they used without further purification. Ethyl cyanoacetate (98%), 1-naphthaldehyde (98%), hydrazine hydrate (86%), methanol (99%), ethanol absolute (99.9%) and carbon disulfide (99%), were provided by Sigma-Aldrich, USA. Polyvinyl chloride (PVC) was provided by Hanwha (KM-31, Seoul, Korea).
3.1 Synthesis (E)-2-(5-mercapto-4-((naphthalene-1-ylmethylene)amino)-4H-1,2,4-triazol-3-yl)acetonitrile
A mixture of (0.0006 mol, 0.1 g) 2-(4-amino-5-mercapto-4H-1,2,4-triazol-3-yl)acetonitrile were synthesized as previously reported [9], and 1-naphthaldehyde (0.0006 mol) in ethanol (10 mL) containing glacial acetic acid was refluxed for 4–6 h. After cooling, an orange solid appeared which was collected by filtration and recrystallized from ethanol to give Schiff base as a final sample.
3.2 Preparation of PVC-ligand films
0.25 g PVC and 0.05 g Schiff base in tetrahydrofuran (THF) solvent were mixed and refluxed for 3 h. The prepared PVC solution was casted onto cleaned glass plate and the films have been generated by implementing the evaporation method at 25 °C. Digital vernier was used to maintain 40 μm as a thickness for PVC-L films.
3.3 Preparation of PVC-ligand-Cu(II) complex films
0.3 g of synthesized modified polymer (PVC-L) and 0.05 g of copper chloride were dissolved in 5 mL of THF. The mixture was refluxed for 3 h in order to form the complex PVC-L-Cu(II) by evaporation technique for 24 h at room temperature.
3.4 UV light exposure
The achieved samples of PVC films mention above were exposed to UV light generated from QUV tester (313 nm as λmax, while light intensity was set to be 6.43 × 10−9 ein dm−3 s−1) for 300 h as irradiation testing.
4 Results and discussion
Schiff base was synthesized as shown in Scheme 1. Reaction of 2-(4-amino-5-mercapto-4H-1,2,4-triazol-3-yl)acetonitrile and 1-naphthaldehyde in boiling ethanol containing acetic acid as a catalyst for 4–6 h gave Schiff base (Scheme 1) with melting point range (190–191) °C in 59% yield.
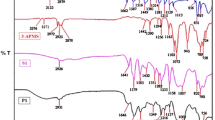
The structure of Schiff base was confirmed by FTIR spectroscopy. The FTIR spectra of Schiff base shows the presence of intense absorption bands at 1578 cm−1 can be attributed to the CH=N bond, also indicate disappearance of (C=O) and NH2 groups absorptions. The FTIR spectra of Schiff base shows (C–H) aromatic, (C–H) aliphatic, C=C and C-S absorptions at 3108, (2872–2984), 1508 and 773, respectively.
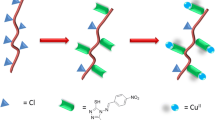
1H-NMR spectroscopy was used to record the proton nuclear magnetic resonance (1H-NMR; 300 MHz) spectra for 4 in DMSO-d6, it showed signals at (3.75) ppm for CH2, signals at (7.91–8.11) for 6H of two phenyl group, (8.21) ppm for CH=N, and (10.18) ppm for SH. Recently, scientists ought to modify PVC, via creation aromatic and heterocyclic moieties thru reaction of halogen displacement. Generally thought, the displacement of facial chlorine from PVC introduces the potential of anchoring of ligands simplicity to PVC matrix and the immobilized transition metal complexes [10]. Figure 1 confirmed the changed in the polymeric film. Coordination of the ligand by SH with PVC chains.
The SN2 is the mechanism for the preparation of the modified PVC by aromatic and heterocyclic moieties in case of introducing of halogen as displacement agent. The photodegradation degree of polymeric films was monitored by FTIR spectra in the range (4000 − 400) cm−1. The FTIR spectra of modified PVC-L film show (C–H) stretching of –CH2– at 2885 cm−1, (C–Cl) stretching at 630 cm−1, stretching vibration of (CH=N) at 1687 cm−1 and (C–H) bending of –CH2– at 1385 cm−1. The FTIR spectra of PVC-L-Cu(II) show (C–H) stretching of –CH2– at 2937 cm−1, (C–Cl) stretching at 697 cm−1, stretching vibration of (CH=N) at 1687 cm−1 and (C–H) bending of –CH2– at 1437 cm−1.
The most common oxygenated products are aldehydes, chloroketones, chlorocarboxylic acids, and acid chlorides. The carbonyl-containing fragments usually can be detected using FTIR pattern upon irradiation. Therefore, PVC films were exposed for UV light irradiation for different time periods and the FTIR spectra were subsequently recorded. The FTIR spectra for PVC (blank) before under three conditions (Indeed beside the original sample): (0 h) and after irradiation (100, 200, and 300 h) are shown in Fig. 2. It clearly shows that the intensity of the peak at 1722 cm−1 corresponding to carbonyl group (C=O) vibration became apparent upon irradiation, and its intensity increased as the irradiation time increased. PVC films blended with Cu(II) chelates suggests the increase of photostabilization [10].
The intensity of the C=O group in the FTIR spectra was monitored upon irradiation which has been compared to a standard peak of 1328 cm−1 that corresponds with C–H bond in the CH2 units of PVC. Films blended with Cu(II) chelate acts as photostabilizer. As Fig. 3 shows IC=O changes occurred for PVC films due to irradiation exposure. Carbonyl index (IC=O) was used to identify PVC films photo-oxidation degree using below formula:
where AC=O is the carbonyl group peak area, and ARef. is the C–C bonds peak area corresponding (1328 cm−1).
Accelerated degradation of PVC and the mechanism of occurred degradation under wavelength 313 nm irradiation light have been carefully studied. This study shows if degradation can take place under accelerated sunlight, this method will become an excellent one for the degradation of waste plastics, which is mainly required for green chemistry.
The PVC films were weighted during irradiation process. The measurements were performed every 50 h till reach to 300 h after removing them from the climatic chamber, using an analytical balance. The presented data proves the weight loss for blank PVC film was higher in compared to PVC films blended with Cu(II) which exhibit least weight loss, as displayed in Fig. 4.
The SEM examines the effect of UV irradiation on the surface morphology of PVC films [11]. Chemical modification on PVC films can be done through chlorine atom nucleophilic substitution reaction inside certain kind of solution which works as giver, as have been reported by other scientists. The surface morphology of PVC films were examined using SEM, the results were shown in Fig. 5. UV light has been supplied on both of PVC (blank) film and PVC-L-Cu(II) films for irradiation with an (λmax = 313 nm) for 300 h. SEM images provided the necessary information about particle shape and size which expressed that the for the non-irradiated PVC (blank) had smooth and neat surfaces with a high degree of homogeneity [12, 13].
For PVC-L contains Copper(II) chloride film after UV irradiation for 300 h, the results shown that fabrication of porous PVC material is due to the cross-linking and evolution of hydrogen chloride and other volatile products.
The SEM images of PVC films revealed forming of honeycomb-like morphology which we can conclude safely this roughness at the film surface is due to the interaction/coordination happened between the PVC-Schiff base blend and Cu(II) ion as a consequence of pass-linking. The images designate that the PVC mixture changed into well suited with identical matrix [14]. Obviously, for each Schiff base and CuCl2 had been often distributed inside the chains of the PVC sample. However, numbers of round pores were noticed on the surface probable as a result of the accelerated evaporation of tetrahydrofuran which been introduced to the process of the film production as a solvent. Knowing the different or variation in what is known as deriving forces during the phase separation, the pore size turned into varying [15]. Meanwhile, PVC and Cu(II) Schiff morphology has changed to the hexagonal profile. High stability of the treated PVC samples was achieved as effect of that hexagonal shape and the Cu2+ ion which might act as transmit stabilizers [16]. Porous shape was endorsed to Cu2+ ions coordination and incorporation on the sample surface and more within the polymer matrix. Commonly, high surface area comes as consequence of the porous nature existence on the film surface with decreasing of crystalline size [17]. It has cited by number of researcher that the shape of the honeycomb is strongly depending number of factors such as solvent type, polymer facet-chain period and the polymer concentration [17]. The SEM topography has been intensively analyzed to study the impact of UV light exposure on the PVC film samples as an effective tool and diagnose the particles size the their shapes [18, 19]. The EDX technique is sufficient technique to detect the elemental composition of polymeric materials [12], Fig. 6 shows that the PVC-L-Cu(II) film exhibits appearance of new band that related to the Cu(II).
5 Conclusion
In summary, we investigated a simple method for the preparation of modified poly(vinyl chloride). A Schiff base containing the 1,2,4-triazole moiety was synthesized and used to modify the polymer. PVC film blended with Cu(II) chelates was irradiated with UV light to observe a number of honeycomb-like on the surface. The PVC-L-Cu(II) was found to be the most effective in photostabilization process.
References
Kilduft JE, Mattaraj S, Pieracci JP (2000) Photochemical modification of poly(ether sulfone) and sulfonated poly(sulfone) nanofiltration membranes for control of fouling by natural organic matter. Desalination 132:133–142
Yang C, Mei Li X, Gilron J, Feng Kong D, Yin Y, Oren Y, Linder C, He T (2014) CF4 plasma-modified super hydrophobic PVDF membranes for direct contact membrane distillation. J Membr Sci 456:155–161
Liu SX, Kim JT, Kim S (2008) Effect of polymer surface modification on polymer–protein interaction via hydrophilic polymer grafting. J Food Sci 73:E143–E150
Qiu J, Zhang Y, Shen Y, Zhang Y, Zhang H, Liu J (2010) Hydrophilic modification of microporous polysulfone membrane via surface-initiated atom transfer radical polymerization of acrylamide. Appl Surf Sci 256:3274–3280
Yu M, Zhang BW, Deng B, Li LF, Xie LD, Lu XF, Sheng KL, Li JY (2010) Introducing reactive groups into polymer chains by radiation induced grafting technique. Plast, Rubber Compos 39:79–82
Ebewele RO (2000) Polymer science and technology. CRC Press LLC, New York
Tosh B, Routray CR (2013) Graft copolymerization of methyl methacrylate onto cellulose in homogeneous medium-effect of solvent and initiator. Int J Chem Nucl Metall Mater Eng 7:89–96
Sharma RK, Kumar S (2013) Synthesis and characterization of graft copolymers of mulberry silk fiber with vinyl binary monomers. Adv Appl Sci Res 4:41–51
Yousif E, Ahmed DS, Ahmed A, Abdallh M, Yusop RM, Mohammed SA (2019) Impact of stabilizer on the environmental behavior of PVC films reinforced 1,2,4-triazole moiety. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-019-05784-w
Yousif E, Asaad N, Ahmed DS, Mohammed SA, Jawad AH (2019) A spectral, optical, microscopic study, synthesis and characterization of PVC films containing Schiff base complexes. Baghdad Sci J 16:56–60
Nikafshar S, Zabihi O, Ahmadi M, Mirmohseni A, Taseidifar M, Naebe M (2017) The effect of UV light on the chemical and mechanical properties of a transparent epoxy-diamine system in the presence of an organic UV absorber. Materials 10:180
Hashim H, El-Hiti GA, Alotaibi MH, Ahmed DS, Yousif E (2018) Fabrication of ordered honeycomb porous poly(vinyl chloride) thin film doped with a Schiff base and nickel(II) chloride. Heliyon 4:e00743
Alotaibi MH, El-Hiti GA, Hashim H, Hameed AS, Ahmed DS, Yousif E (2018) SEM analysis of the tunable honeycomb structure of irradiated poly(vinyl chloride) films doped with polyphosphate. Heliyon 4:e01013
Wu TM, Lin YW, Liao CS (2005) Preparation and characterization of polyaniline/multiewalled carbon nanotube composites. Carbon 43:734–740
Rhoo HJ, Kim HT, Park JK, Hwang TS (1997) Ionic conduction in plasticized PVC/PMMA blend polymer electrolytes. Electrochim Acta 42:1571–1579
Rahman MYA, Ahmad A, Lee TK, Farina Y, Dahlan HD (2011) Effect of ethylene carbonate(EC) plasticizer on poly(vinyl chloride)-liquid 50% epoxidised natural rubber (LENR50) based polymer electrolyte. Mater Sci Appl 2:817–825
Huh M, Gauthier M, Yun S (2016) Honeycomb structured porous films prepared from arborescent graft polystyrenes via the breath figures method. Polymer 107:273–281
Amiri O, Salavati-Niasari M, Mir N, Beshkar F, Saadat M, Ansari F (2018) Plasmonic enhancement of dye-sensitized solar cells by using Au-decorated Ag dendrites as a morphology-engineered. Renew Energy 125:590–598
Chiuzbăian SG, Brignolo S, Hague CF, Delaunay R, Guarise M, Nicolaou A, Yang Z, Zhou H, Mariot JM (2017) Spectroscopic evidence for super exchange in the ferromagnetic spinel FeCr2S4. J Phys Chem C 121:21935–21944
Acknowledgements
The authors would like to extend their appreciation to Al-Nahrain University for continued support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yousif, E., Ahmed, D.S. Poly(vinyl chloride) reinforced Schiff base as an eco-friendly alternative to conventional PVC. SN Appl. Sci. 1, 955 (2019). https://doi.org/10.1007/s42452-019-0993-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0993-3