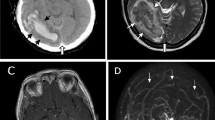
Abstract
Neurological manifestations related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection may involve both peripheral and central nervous systems, including acute ischemic stroke and cerebral venous sinus thrombosis (CVST). Hereby, we present an adult patient with post-vaccination breakthrough COVID-19 infection and CVST, treated with venous mechanical thrombectomy (MT). The patient manifested continuous tonic-clonic seizures, after an insidious presentation with headache and left-sided weakness. SARS-CoV-2 testing was positive, despite full vaccination, using two approved mRNA platforms. Factor V Leiden polymorphism was detected. The patient was initially managed with anticoagulation, followed by MT with a positive response. We provided a comparison to similar cases of COVID-19-associated CVST undergoing mechanical thrombectomy. High index of suspicion and prompt diagnosis are extremely important to ensure immediate hospitalization and therapy, since CVST associated with either vaccines or COVID-19 seems to evolve rapidly and with a high mortality rate. Even a breakthrough infection may present severe vascular complications. In addition, evaluation of acquired and hereditary thrombophilia may be beneficial in acute phase, also without a previous history of thrombosis. Clinicians should start early medical treatment and additionally consider the endovascular approach as an optimistic choice in refractory CVST related to SARS-CoV-2 infection.

Similar content being viewed by others
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Code Availability
Not applicable.
References
Baldini T, Asioli GM, Romoli M, Carvalho Dias M, Schulte EC, Hauer L, et al. Cerebral venous thrombosis and severe acute respiratory syndrome coronavirus-2 infection: a systematic review and meta-analysis. Eur J Neurol. 2021;28(10):3478–90.
Accorsi EK, Britton A, Fleming-Dutra KE, Smith ZR, Shang N, Derado G, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. JAMA. 2022;327(7):639–51. https://pubmed.ncbi.nlm.nih.gov/35060999/
Demchuk AM, Scott Burgin W, Christou I, Felberg RA, Barber PA, Hill MD, et al. Thrombolysis in brain ischemia (TIBI) transcranial Doppler flow grades predict clinical severity, early recovery, and mortality in patients treated with intravenous tissue plasminogen activator. Stroke. 2001;32(1):89–93.
Ferro JM, Bousser MG, Canhão P, Coutinho JM, Crassard I, Dentali F, et al. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis – endorsed by the European Academy of Neurology. Eur J Neurol. 2017;24(10):1203–13.
Ostovan VR, Foroughi R, Rostami M, Almasi-Dooghaee M, Esmaili M, Bidaki AA, et al. Cerebral venous sinus thrombosis associated with COVID-19: a case series and literature review. J Neurol. 2021;268(10):3549–60.
Sajjad A, Khan AF, Jafri L, Kamal AK. Successful endovascular mechanical thrombectomy in anticoagulation-resistant COVID-19 associated cerebral venous sinus thrombosis. BMJ Case Rep. 2021;14(12) https://pubmed.ncbi.nlm.nih.gov/34972772/
Cavalcanti DD, Raz E, Shapiro M, Dehkharghani S, Yaghi S, Lillemoe K, et al. Cerebral venous thrombosis associated with COVID-19. Am J Neuroradiol. 2020;41(8):1370–6.
Marshall JC, Murthy S, Diaz J, Adhikari N, Angus DC, Arabi YM, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20(8):e192–7.
Tobaiqy M, Maclure K, Elkout H, Stewart D. Thrombotic adverse events reported for Moderna, Pfizer and Oxford-AstraZeneca COVID-19 vaccines: comparison of occurrence and clinical outcomes in the EudraVigilance Database. Vaccines. 2021;9(11):1326. https://pubmed.ncbi.nlm.nih.gov/34835256/
Kaplonek P, Cizmeci D, Fischinger S, Collier A, Suscovich T, Linde C, et al. mRNA-1273 and BNT162b2 COVID-19 vaccines elicit antibodies with differences in Fc-mediated effector functions. Sci Transl Med. 2022;14(645):eabm2311.
Atmar RL, Lyke KE, Deming ME, Jackson LA, Branche AR, El Sahly HM, et al. Homologous and heterologous COVID-19 booster vaccinations. N Engl J Med. 2022;386(11):1046–57. https://doi.org/10.1056/NEJMoa2116414.
Accorsi EK, Britton A, Fleming-Dutra KE, Smith ZR, Shang N, Derado G, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. JAMA. 2022;327(7):639–51. https://jamanetwork.com/journals/jama/fullarticle/2788485
Rodriguez-Sevilla JJ, Güerri-Fernádez R, Bertran RB. Is there less alteration of smell sensation in patients with omicron SARS-CoV-2 variant infection? Front Med. 2022;12(9):1044.
Narvel H, Kaur A, Seo J, Kumar A. Multisystem inflammatory syndrome in adults or hemophagocytic lymphohistiocytosis: a clinical conundrum in fully vaccinated adults with breakthrough COVID-19 infections. Cureus. 2022;14(2):1–12.
Vogel TP, Top KA, Karatzios C, Hilmers DC, Tapia LI, Moceri P, et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2021;39(22):3037–49.
Acknowledgements
We thank the patient and the patient’s family for their kind cooperation. We would like to thank all professionals involved in stroke care during the pandemic.
Author information
Authors and Affiliations
Contributions
FGi, FGr, and ATe contributed to the study design. F Gi, AG, and LF performed data collections. ATe and DI performed imaging analysis. AAC, MV, and OB performed endovascular treatment. FGi and AG wrote the article. FGr reviewed and critiqued the manuscript. ATo, PLS, and SLV supervised the research.
Corresponding author
Ethics declarations
Ethics Approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The paper does not report on primary research. Our analysis looked retrospectively at outcomes for a large cohort of patients treated. All data analyzed were collected as part of routine diagnosis and treatment.
Consent to Participate
A handwritten signature was obtained from the patient.
Consent for Publication
A handwritten signature was obtained from the patient.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on COVID-19
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Giammello, F., Grillo, F., Tessitore, A. et al. Management of Severe Cerebral Venous Sinus Thrombosis After Post-vaccination Breakthrough COVID-19 Infection: A Case Report and Review of the Literature. SN Compr. Clin. Med. 5, 167 (2023). https://doi.org/10.1007/s42399-023-01506-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s42399-023-01506-z