Abstract
Cancer patients are a vulnerable population in the current coronavirus disease 2019 (COVID-19) outbreak. The impact of immune checkpoint inhibitors (ICIs) on the outcomes of COVID-19 infection in cancer patients remains largely unclear. We retrospectively investigated all solid cancer patients who received at least one cycle of ICIs at a single institution between August 2020 and August 2021. All stage IV solid cancer patients who were on or ceased ICI treatment when diagnosed with COVID-19 were eligible. All COVID-19 infections were confirmed by RT-PCR. Risk factors for hospitalization, severe symptoms, and death were analyzed. A total of 56 patients were included in our study. Twenty (35.7%) patients require hospitalization, 12 (21.4%) developed severe symptoms, and 10 (17.9%) died from COVID-19 infection. ICI treatment was interrupted in 37 patients (66.1%), 24 of whom (64.9%) had treatment resumed. Eight (80%) COVID-19-related death occurred in unvaccinated individuals. Reinfection occurred in seven patients (12.5%), and three of them died from their second COVID-19 infection. Factors associated with hospitalization were high Charlson comorbidity score (OR 1.56, 95% CI 1.10–2.23, p = 0.01) and lymphocyte ≤ 1500 mm3 (OR 10.05, 95% CI 2.03–49.85, p = 0.005). Age, chemoimmunotherapy, and ICI treatment duration were not associated with increased risk of hospitalization, severe symptoms, or COVID-19-related mortality. ICI therapy does not impose an increased risk for severe COVID-19 infection in stage IV cancer patients. Vaccination should be encouraged among this population. Clinicians should be cognizant of a potential worse outcome in COVID-19-reinfected patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global coronavirus disease 2019 (COVID-19) pandemic has brought about a significant burden on the health care system, posing challenges to cancer patient care [1, 2]. Compared to those without malignancies, cancer patients are more likely to develop severe symptoms and worse outcomes, owing to comorbidity-related and overall immunosuppressive status caused by both cancer and anticancer treatment such as chemotherapy [3, 4].
Immune checkpoints consist of both stimulatory and inhibitory pathways that regulate the immune system. Programmed cell death 1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T lymphocyte-associated protein-4 (CTLA-4) are immune checkpoint proteins that play pivotal roles in tumor evasion from antitumor immunity [5, 6]. By blocking these inhibitory checkpoint proteins, the immune checkpoint inhibitors (ICIs) promote T cells to attack cancer cells [7]. Over the last decade, ICIs including PD-1/PD-L1 inhibitors and CTLA-4 inhibitors have revolutionized cancer management and are fundamental to the management of various types of advanced stage solid cancers, including lung cancer, melanoma, and kidney cancer [8,9,10].
Data regarding the ICI impact on the risk of severe infection and mortality in COVID-19-infected cancer patients are rather limited. Theoretically, the ICIs could either alleviate or aggravate COVID-19 severity. On the one hand, it is documented that during viral infections, blocking PD-1 pathway enhances viral elimination by preventing or mitigating T cell exhaustion, avoiding the suppression of antiviral immune responses [11, 12]. Studies indicate that COVID-19 may cause T cell exhaustion by continuous PD-1/PD-L1/CTLA-4 expression, and ICI treatment may exert antiviral effect by counteracting the COVID-19-induced T cell immunologic impairment [13,14,15]. Currently, a few clinical trials (e.g., NCT04335305, NCT04343144, NCT04413838, NCT04356508, NCT04333914, and NCT04268537) have been registered at ClinicalTrials.gov to examine the ICI efficacy in COVID-19-infected patients [16]. On the other hand, ICIs have been associated with a spectrum of autoimmune toxic effects, termed immune-related adverse events (irAEs) [17, 18], including immune-related pneumonitis. COVID-19 is characterized by increased cytokine production. Cytokine release syndrome (CRS) has been reported in patients receiving ICIs as well as those infected with COVID-19 [19,20,21]. The hyperimmune condition associated with both the ICI treatment and COVID-19 may simultaneously cause an adverse immune hyperactivation. Moreover, the potential overlap between immune-related pneumonitis and COVID-19-associated interstitial pneumonia may have a possible synergistic effect in causing the lung damage in ICI-treated cancer patients [19]. Nevertheless, immunosuppressive therapies required for irAE treatment can inhibit antiviral immune response and potentially increase the risk of COVID-19 infection.
It remains unclear whether ICIs are beneficial in treating COVID-19 infection by exerting antiviral immune response, or they may play a detrimental role by causing excessive inflammation leading to adverse outcomes in COVID-19-infected patients. Therefore, we carried out this retrospective study to investigate the impact of ICI treatment on the severity of COVID-19 in cancer patients.
Materials and Methods
We retrospectively investigated all adult solid cancer patients who received at least one cycle of ICIs (PD-1/PD-L1/CTLA4 inhibitors) either as monotherapy or as multi-agent therapy at AdventHealth Orlando infusion center between August 1, 2020, and August 31, 2021. All stage IV cancer patients who were on or ceased ICI treatment when diagnosed with COVID-19 were eligible. Combinations of ICI with chemotherapy or vascular endothelial growth factor inhibitors were allowed. Exclusion criteria included patients with prior ICI treatment who were on non-ICI anticancer treatment (chemotherapy, target therapy, and radiotherapy) at the time of COVID-19 diagnosis and patients who were tested positive for COVID-19 before their first dose of ICIs. All patients were tested for COVID-19 by reverse transcription polymerase chain reaction (RT-PCR) during each treatment cycle per institutional policy. Patients were deemed to have COVID-19 infection if an RT-PCR test from the nasopharyngeal swab was positive for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).
Patient records were individually reviewed to identify eligible patients. We collected data regarding patient demographics, comorbidities, Eastern Cooperative Oncology Group (ECOG) performance status score, and body mass index (BMI) at the time of COVID-19 infection, cancer histology, ICI therapy-related data, COVID-19 vaccination-related data, COVID-19 laboratory data (in those who had laboratory tests available within 3-days of COVID-19 diagnosis), COVID-19 treatment data, and survival outcomes related to COVID-19 infection.
The COVID-19 severity was defined as mild for cases not requiring supplemental oxygen and not requiring hospitalization, moderate for cases requiring hospitalization but not meeting criteria for severe cases, and severe for cases requiring high-flow oxygen therapy, intensive care unit (ICU) admission, need for mechanical ventilation including noninvasive, and invasive mechanical ventilation, or death due to COVID-19. The time to COVID-19 infection was defined as the time between the day of ICI therapy initiation and the day of positive COVID-19 test. Follow-up time since the ICI initiation was defined as the time from the start of the ICI therapy to the date of death or last available visit. Follow-up time since the COVID-19 diagnosis was defined as the time from the date of positive COVID-19 RT-PCR result to the date of death or last available visit. The minimal follow-up duration for alive patients was 30 days since the COVID-19 diagnosis. The censor date was set at March 10, 2022. This study was approved by the Institutional Review Board at AdventHealth Orlando.
Statistical Analyses
Differences between the hospitalized and the non-hospitalized groups were analyzed using Student’s t test for continuous variables and Chi-square or Fisher’s exact test for categorial variables, as applicable. Univariate logistic regression were used to assess the risk factors associated with COVID-19-related hospitalization, severe COVID-19 symptoms, and COVID-19-related death. Due to limited number of events, multivariate analysis was not performed. OR (odds ratio) and associated 95% CI (confidence interval) were reported. All p values were two-sided. The differences between groups were considered statistically significant if p < 0.05. All analyses were done using GraphPad Prism and R version 4.0.
Results
Demographics and Clinical Characteristics of the Patients
From August 1, 2020, to August 31, 2021, 590 cancer patients received at least one cycle of ICI. Among them, 82 patients (13.9%) tested positive for COVID-19 with the earliest infection occurring on April 21, 2020, and the latest infection on February 8, 2022. In the 82 COVID-19-positive patients, 26 patients were excluded due to COVID-19 infection onset prior to ICI initiation (nine patients), non-stage IV status (four patients), or actively receiving other types of therapy after the cessation of ICI treatment when diagnosed with COVID-19 (13 patients). Finally, a total of 56 patients with stage IV malignant solid tumors were included that formed the basis of our study analysis.
Table 1 shows the demographic and clinical characteristics of the 56 patients. The median patient age was 67 years (range, 41–91), and 62.1% cases were female. A total of 32 patients (57.1%) were current or former smokers. The most common cancer subtype was thoracic cancer (35.7%), followed by genitourinary (21.4%) and gynecologic cancers (19.6%). At least one of the specified comorbidities were present in 85.7% patients, including chronic obstructive pulmonary disease (COPD), non-COPD lung disease, coronary artery disease, hypertension, congestive heart failure, diabetes, and chronic kidney disease stage 3 and above. All but two patients were actively receiving ICI treatment when infected with COVID-19. Most patients (91.2%) received PD-1 or PD-L1 inhibitor monotherapy including pembrolizumab (53.6%) and nivolumab (17.9%), followed by atezolizumab (14.3%) and durvalumab (5.4%). Thirteen patients (23.2%) had chemotherapy within 3 months prior to COVID-19 diagnosis, of which 12 patients (21.4%) were on active chemoimmunotherapy when infected with COVID-19.
The majority of patients were treated in the outpatient setting (non-hospitalized group, n = 36, 64.3%), while 20 patients (hospitalized group, 35.7%) required hospital admission including four (7.1%) ICU admissions for COVID-19 management. The baseline characteristics of patients who were hospitalized were similar to those who weren’t hospitalized in terms of age, gender, ethnicity, ECOG performance status, cancer histology, ICI type, smoking status, and obesity. Hospitalized patients had a significantly higher number of comorbidities (p = 0.02) and Charlson comorbidity score (p = 0.004).
COVID-19 Diagnosis and Management
The median time between the last ICI dose to COVID-19 diagnosis was 9.5 days (range, 0–398). All but four patients (92.9%) were on active ICI treatment when diagnosed with COVID-19. Thirty patients (53.6%) were symptomatic at the time of COVID-19 diagnosis, including 20 out of 20 hospitalized patients (100%) and 10 out of 36 non-hospitalized patients (27.8%).
Of the 20 hospitalized patients, half of them chose to do not resuscitate (DNR) (n = 8, 40%) or limited to non-invasive mechanical ventilation only (n = 2, 10%); therefore, they were not intubated or admitted to the ICU. Twelve patients (60%) developed severe symptoms. Oxygen therapy was administered to 18 patients, including low-flow delivery in nine patients (45%), high-flow delivery in seven patients (35%), and mechanical ventilation in two patients (10%). None of these patients received extracorporeal membrane oxygenation.
Of the entire cohort, systemic corticosteroid was given to 20 patients (35.7%); antibiotics and antiviral agents were given to 21 (37.5%) and 17 patients (30.4%), respectively; tocilizumab, baricitinib, and convalescence plasma were given to three (5.4%), one (1.8%), and three (5.4%) patients, respectively.
ICI Interruption and Resumption
In the entire cohort, ICI was interrupted in 37 patients (66.1%), 24 of whom (64.9%) had resumed at the time of data cut-off. A significantly higher ICI interruption rate was seen in the hospitalized patients (17 of 20, 85%), while in the non-hospitalized group, 55.6% (20 of 36) had ICI interrupted. The ICI resumption rate was also lower in the hospitalized group (35.3% vs. 90%, p = 0.001). Of all the asymptomatic patients in the non-hospitalized group, 12 of 26 (46.2%) cases continued their ICI treatment without interruption, while 14 out of 26 patients (53.8%) had ICI interrupted and later resumed.
Vaccination
Twenty patients (35.7%) received COVID-19 vaccination prior to COVID-19 infection. Twenty unvaccinated patients later received their COVID-19 vaccine after recovery from COVID-19 infection, which increased the total vaccination patient number to 40 (71.4%). Although the rates of vaccination prior to COVID-19 infection between hospitalized and non-hospitalized group were not statistically significant, in the 10 patients whose primary cause of death was COVID-19 infection, eight (80%) were unvaccinated.
Re-infection
Of the 56 patients, seven patients (12.5%) had recurrence of positive COVID-19 test results after the initial COVID-19 infection (Table 2). All of them had negative COVID-19 RT-PCR test results in between the initial COVID-19 infection and the second positive COVID-19 test, with the median number of negative tests 4 (range, 2–10) and the median time in between the initial infection and the second positive test 277 days (range, 153–406). Four of them (57.1%) eventually died, and COVID-19 infection was the primary cause of death in three cases (42.9%). Two patients with stage IV non-small cell lung cancer (NSCLC) were hospitalized for both episodes, and unfortunately, both died from the second COVID-19 infection. The third patient who died from COVID-19 infection was diagnosed with stage IV renal cell carcinoma. She became symptomatic during her second COVID-19 episode and was hospitalized for the second episode.
Survival Outcomes
With a median follow-up duration of 420 days (range, 56–2105) since the ICI initiation and 186 days (range, 11–580) since the COVID-19 diagnosis, 15 deaths (26.8%) occurred in the entire cohort, of which COVID-19 was the primary causes of death in 10 patients (17.9%) (Table 3). Of the 10 COVID-19-related deaths, the median time from the COVID-19 diagnosis to death was 16 days (range, 2–59), nine patients (90%) had cancers involving lungs either as primary lung cancers or as lung metastases. Hospitalization due to COVID-19 infection was significantly associated with the increasing mortality rates. While the mortality for patients who received outpatient management for COVID-19 was 8.3% (3 of 36), 12 out of 20 patients (60%) who were hospitalized for COVID-19 died.
Factors Associated with Hospitalization, Severe COVID-19 Disease, and Death
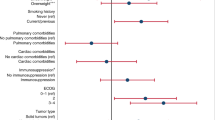
As shown in Table 4, in univariate analysis, higher Charlson comorbidity score (OR 1.56, 95% CI 1.10–2.23, p = 0.01) and lymphocyte count ≤ 1,500/mm3 (OR 10.05, 95% CI 2.03–49.85, p = 0.005) were significantly associated with the increased risk of hospitalization, while the time from last ICI to COVID-19 diagnosis, ICI treatment duration, ICI treatment cycles, and chemoimmunotherapy were not associated with COVID-19-related hospitalization.
In univariate analysis, lymphocyte ≤ 1,500 mm3 was also associated with a higher risk of severe COVID-19 symptoms (OR 9.17, 95% CI 1.09–77.23; p = 0.04) (Table 4). Higher Charlson comorbidity score, ICI cycles prior to COVID-19 diagnosis, ICI treatment duration prior to COVID-19 diagnosis, and time from last ICI to COVID-19 diagnosis were not associated with severe symptoms or COVID-19-related death. Due to limited number of events, multivariate analysis was not performed.
Discussion
The host immune system is pivotal in the outcome of COVID-19 infection. Study has shown that ICI-treated cancer patients may be more immunocompetent compared to the chemotherapy-treated patients [16, 22]. Immunotherapy may be able to restore the cellular immunocompetence, helping clear SARS-CoV-2 [23]. Meanwhile, the potential synergy between ICI mechanisms and COVID-19 pathogenesis on inducing adverse immune hyperactivation and the possible overlap between COVID-19 pneumonia and immune-related pneumonitis are of particular concern. In this single-center retrospective study, we investigated the impact of ICI treatment on 56 stage IV solid malignant tumor patients with laboratory-confirmed COVID-19 infection.
Almost half of our study population were asymptomatic, which was higher than reported by others [2, 24,25,26]. In our experience, 17.9% patients had severe COVID-19 symptom, which was lower than reported 33% in the GCO-002 CACOVID-19 study [27]. The observed higher proportion of asymptomatic patients and lower proportion of severe COVID-19 cases in our cohort was likely related to the fact that patients in our cohort were repeatedly tested for COVID-19 via RT-PCR, making our data more representative of the true COVID-19 severity in the ICI-treated advanced stage cancer patients, while most studies were conducted early in COVID-19 pandemic when the screening in cancer patients was not universal, which may lead to selection bias and a potentially higher proportion of symptomatic patients.
Despite COVID-19 infection, ICI was not interrupted in about one-third of the patients (19 out of 56) in our study, with most of them asymptomatic. Eighteen out of the 20 ICI-interrupted non-hospitalized patients (90%) later resumed their ICI treatment. Continuous ICI therapy without interruption may be a feasible strategy for asymptomatic COVID-19-infected patients, while proper caution should be maintained to mitigate the spread of infection from asymptomatic patients to others.
In our study, seven patients (12.5%) had reinfection, and three died from the second COVID-19 infection, which consisted 42.9% of the reinfected subgroup. Of the three deceased patients, two were admitted for both the initial and the second COVID-19 infection episodes. Cancer patients may be tested positive for COVID-19 repeatedly due to the failure to clear an initial infection. We were not able to analyze the viral genome to further confirm if the two infection episodes were caused by different variants of SARS-CoV-2. Although the possibility of reactivation of prior infection due to incomplete clearance of SARS-CoV-2 cannot be ruled out, all the seven patients had several negative tests in between the two positive RT-PCR test results, making reinfection more likely. Solid cancer patients are potentially more vulnerable to reinfection with COVID-19 compared to the general population, owing to the impaired immune response to the virus. Clinicians should be aware of the potential high mortality rate among this subgroup of ICI-treated cancer patients, especially among those who had prior history of COVID-19-related hospitalization and prioritize their care whenever possible.
About one third of the COVID-19-infected patients in our study were admitted to hospital, in line with the rate reported by Rogiers et al. but lower than reported by earlier studies [2, 28]. This is likely related to a higher proportion of asymptomatic patients in our study since patients were regularly tested with RT-PCR by our infusion center. The ICU admission rate in our study was 7.1%, which was relatively lower compared to the reported incidence of 14.5% [29]. Since in our study population all cases were at advanced stage cancer, it was not surprising to see a high full and partial DNR rate in this population (50% of the hospitalized group and 90% of those who died from COVID-19 infection), which may explain the low ICU admission rate.
In line with other reports [2, 27, 28, 30], our study showed that high Charlson comorbidity score is associated with increased risk of hospitalization, and lymphopenia is a predictor for both COVID-19-related hospitalization and severe COVID-19. On the other hand, unlike prior study but similar to Rogiers study [2, 26, 31, 32], we did not notice any association between older age with a worse outcome in patients infected with COVID-19, which might be related to the fact that our study population consisted of an elderly population with a median age of 67 years. Different from Rogiers et al. 2, one-fifth of the patients in our study were actively receiving chemoimmunotherapy when diagnosed with COVID-19. It has been reported that chemotherapy within 4 weeks of COVID-19 infection was associated with COVID-19-related hospital mortality [33]. However, in our study, using univariate analysis, we did not find any association between chemoimmunotherapy treatment with increased hospital admission, severe symptoms, or COVID-19-related death. Our relatively small patient size might be the explanation to this discrepancy. However, differences may exist in the extent of immunosuppression induced by chemotherapy alone compared with chemoimmunotherapy, contributing to the observed discrepancy.
Data regarding the impact of ICI treatment on the survival outcome of cancer patients infected with COVID-19 are limited and conflicting [24, 26,27,28,29, 31, 32, 34,35,36,37]. One study suggested that the history of PD-1 inhibitor exposure was not associated with increased risk of severe COVID-19 disease in lung cancer patients, regardless of the time interval from the last ICI dose received [36]. Similarly, we did not observe any association between the time from the last ICI exposure to COVID-19 diagnosis and hospitalization or severe symptoms. It has been shown that > 70% of PD-1 receptors may be occupied for more than 2 months following last PD-1 inhibitor administration [38], which may partially explain why the time interval from last ICI treatment was not linked to the severity of COVID-19. Wu et al. carried out a small retrospective study involving 11 cancer patients and speculated that the severity of COVID-19 could be associated with the number of ICI cycles received, with those who had three or more cycles more likely to develop severe COVID-19 [39]. However, our study did not suggest ICI exposure duration to be a risk factor of severe COVID-19. On the contrary, we noticed that in the hospitalized group, the median number of ICI cycles patient received before the COVID-19 infection was lower than those who underwent out-patient management, although the difference was not significant (6.5 cycles vs. 10.5 cycles, p = 0.85). In our study, 10 patients (17.2%) died from COVID-19, which was at the lower end of the reported range of the case fatality rate in cancer patients (from 11 to 33%) [2, 3, 24, 27, 33, 40, 41]. This suggests that ICI therapy does not impose an increased risk for worse COVID-19-related outcomes in cancer patients. Our study also suggests the potential protective effect of COVID-19 vaccination, since 80% of COVID-19-related deaths in our cohort occurred in unvaccinated patients. However, due to limited death events, this potential protective effect should be interpreted with caution.
Our study has several limitations. First, it was a retrospective, single-center study with a limited sample size. Second, the case fatality rate, patient characteristics, and survival outcomes were not compared with a control group of patients receiving other anticancer therapies. Third, our study did not evaluate the long-term effect of COVID-19 infection and especially asymptomatic infection on overall survival in ICI-treated cancer patients. Moreover, we were not able to evaluate the effectiveness of vaccination on preventing COVID-19 infection since our study population included only COVID-19-infected patients. Additional studies are desirable to further investigate the benefit of COVID-19 vaccination in patients with advanced stage malignant tumors.
This study has some strengths. The proportion of asymptomatic patients was likely to be underestimated in prior studies, since asymptomatic patients were less likely to be frequently tested and SARS-CoV-2 RT-PCR has a well described false negative rate [42]. The median follow-up duration in our study was 186 days since COVID-19 diagnosis, which was comparably longer than several other studies [2, 24, 27,28,29, 32, 33, 36, 40]. Also, patients in our study were routinely tested for COVID-19 per institutional policy. This allowed us to better evaluate the true prevalence of COVID-19 in our study population and the severity of COVID-19 among stage IV cancer patients. Moreover, unlike other studies where the study population had various stages of cancer with different indications for ICI therapy, the patients in our study all had stage IV disease who received ICI treatment with a palliative intent.
Conclusions
In summary, our study showed COVID-19-related mortality in stage IV cancer patients treated with ICIs was not higher than prior reported mortality rates for cancer patients. The ICI therapy does not impose an increased risk for severe COVID-19 infection in advanced stage cancer patients. A high Charlson comorbidity score and lymphopenia are associated with increased risk of hospitalization in ICI-treated stage IV cancer patients. Vaccination should be encouraged among this population as we noted high proportion of COVID-19-related death in unvaccinated patients. Clinicians should be cognizant of a potential worse outcome in COVID-19-reinfected patients, especially patients who have a history of COVID-19-related hospitalization for their initial COVID-19 infection.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
García LF. Immune response, inflammation, and the clinical spectrum of COVID-19. Front Immunol. 2020;16(11):1441.
Rogiers A, Da Silva IP, Tentori C, et al. Clinical impact of COVID-19 on patients with cancer treated with immune checkpoint inhibition. J Immunother Cancer. 2021;9(1):e001931.
Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783–91.
Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10(7):935–41.
Park R, Winnicki M, Liu E, et al. Immune checkpoints and cancer in the immunogenomics era. Brief Funct Genomics. 2019;18(2):133–9.
Marin-Acevedo JA, Dholaria B, Soyano AE, et al. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol. 2018;11(1):39.
Marin-Acevedo JA, Kimbrough EO, Lou Y. Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol. 202;14(1):1–29
Xiong W, Zhao Y, Du H, et al. Current status of immune checkpoint inhibitor immunotherapy for lung cancer. Front Oncol. 2021;11:704336.
Herrscher H, Robert C. Immune checkpoint inhibitors in melanoma in the metastatic, neoadjuvant, and adjuvant setting. Curr Opin Oncol. 2020;32(2):106–13.
Rini BI, Battle D, Figlin RA, et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of advanced renal cell carcinoma (RCC). J Immunother Cancer. 2019;7(1):1–20.
Schönrich G, Raftery MJ. The PD-1/PD-L1 axis and virus infections: a delicate balance. Front Cell Infect Microbiol. 2019;13(9):207.
Dyck L, Mills KH. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur J Immunol. 2017;47(5):765–79.
Awadasseid A, Yin Q, Wu Y, Zhang W. Potential protective role of the anti-PD-1 blockade against SARS-CoV-2 infection. Biomed Pharmacother. 2021;142:111957.
Aghbash PS, Eslami N, Shamekh A, Entezari-Maleki T, Baghi HB. SARS-CoV-2 infection: the role of PD-1/PD-L1 and CTLA-4 axis. Life Sci. 2021;270:119124.
Mandala M, Lorigan P, De Luca M, et al. SARS-CoV-2 infection and adverse events in patients with cancer receiving immune checkpoint inhibitors: an observational prospective study. J Immunother Cancer. 2021;9(2):e001694.
Vivarelli S, Falzone L, Torino F, et al. Immune-checkpoint inhibitors from cancer to COVID-19: a promising avenue for the treatment of patients with COVID-19. Int J Oncol. 2021;58(2):145–57.
Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management, and surveillance. Nat Rev Clin Oncol. 2019;16(9):563–80.
Choi J, Lee SY. Clinical characteristics and treatment of immune-related adverse events of immune checkpoint inhibitors. Immune Netw. 2020;20(1):e9.
Bersanelli M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy. 2020;12(5):269–73.
Rotz SJ, Leino D, Szabo S, Mangino JL, Turpin BK, Pressey JG. Severe cytokine release syndrome in a patient receiving PD-1-directed therapy. Pediatr Blood Cancer. 2017;64(12):e26642.
Pasrija R, Naime M. The deregulated immune reaction and cytokines release storm (CRS) in COVID-19 disease. Int Immunopharmacol. 2021;90:107225.
Shah NJ, Al-Shbool G, Blackburn M, et al. Safety and efficacy of immune checkpoint inhibitors (ICIs) in cancer patients with HIV, hepatitis B, or hepatitis C viral infection. J Immunother Cancer. 2019;7(1):353.
Isgrò MA, Vitale MG, Celentano E, et al. Immunotherapy may protect cancer patients from SARS-CoV-2 infection: a single-center retrospective analysis. J Transl Med. 2021;19(1):132.
Garassino MC, Whisenant JG, Huang LC, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(7):914–22.
Garde-Noguera J, Fernández-Murga ML, Giner-Bosch V, et al. Impact of SARS-CoV-2 infection on patients with cancer: retrospective and transversal studies in Spanish population. Cancers. 2020;12(12):3513.
Lara OD, O’Cearbhaill RE, Smith MJ, et al. COVID-19 outcomes of patients with gynecologic cancer in New York City. Cancer. 2020;126(19):4294–303.
Lièvre A, Turpin A, Ray-Coquard I, et al. Risk factors for coronavirus disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: a French nationwide cohort study (GCO-002 CACOVID-19). Eur J Cancer. 2020;141:62–81.
Albiges L, Foulon S, Bayle A, et al. Determinants of the outcomes of patients with cancer infected with SARS-CoV-2: results from the Gustave Roussy cohort. Nat Cancer. 2020;1(10):965–75.
Pinato DJ, Zambelli A, Bower M, et al. Clinical portrait of the SARS-CoV-2 epidemic in European patients with cancer. Cancer Discov. 2020;10(10):1465–74.
Bersanelli M, Giannarelli D, De Giorgi U, et al. Symptomatic COVID-19 in advanced-cancer patients treated with immune-checkpoint inhibitors: prospective analysis from a multicentre observational trial by FICOG. Ther Adv Med Oncol. 2020;12:1758835920968463.
Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26(8):1218–23.
Lee LY, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. The Lancet. 2020;395(10241):1919–26.
Yang K, Sheng Y, Huang C, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):904–13.
Qian W, Ye Y, Zuo L, et al. Immune checkpoint inhibitors use and effects on prognosis of COVID-19 infection: a systematic review and meta-analysis. Immunotherapy. 2021;13(15):1271–82.
Klebanov N, Pahalyants V, Murphy WS, et al. Risk of COVID-19 in patients with cancer receiving immune checkpoint inhibitors. Oncologist. 2021;26(5):e898-901.
Luo J, Rizvi H, Egger JV, Preeshagul IR, Wolchok JD, Hellmann MD. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 2020;10(8):1121–8.
Gonzalez-Cao M, Basa MA, Puertolas T, et al. Cancer immunotherapy does not increase the risk of death by COVID-19 in melanoma patients. MedRxiv. 2020. https://doi.org/10.1101/2020.05.19.20106971.
Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti–programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–75.
Wu Q, Chu Q, Zhang H, et al. Clinical outcomes of coronavirus disease 2019 (COVID-19) in cancer patients with prior exposure to immune checkpoint inhibitors. Cancer Commun. 2020;40(8):374–9.
Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. The Lancet. 2020;395(10241):1907–18.
Miyashita H, Mikami T, Chopra N, et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol. 2020;31(8):1088–9.
Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D, et al. False-negative results of initial RT-PCR assays for COVID-19: a systematic review. PLoS One. 2020;15(12):e0242958.
Author information
Authors and Affiliations
Contributions
M.G.: conceptualization, methodology, data collection analyses, investigation, writing, and review.
J.L.: investigation, data collection, data analysis, and writing.
S.Z.: data analysis support and review and editing.
J.Y.: review and editing.
Z.A.: data collection and review.
S.A.: review and writing and editing.
M.M.: conceptualization, methodology, supervision, and review.
M.A.S.: supervision and review.
T.M.: supervision and review.
V.H.: conceptualization, methodology, supervision, and review and editing.
Corresponding authors
Ethics declarations
Ethics Approval
This study was approved by the Institutional Review Board at AdventHealth Orlando.
Consent to Participate
Not applicable.
Consent for Publication
All the authors have read and approved the final draft for publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Covid-19
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, M., Liu, J., Zhou, S. et al. COVID-19 Outcomes in Stage IV Cancer Patients Receiving Immune Checkpoint Inhibitors. SN Compr. Clin. Med. 4, 193 (2022). https://doi.org/10.1007/s42399-022-01277-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s42399-022-01277-z