Abstract
This study aims to investigate the perinatal outcomes in COVID-19 pregnant women with intrahepatic cholestasis of pregnancy (ICP) and elevated liver enzymes. Present study was carried out on pregnant women with COVID-19 between March 11, 2020, and August 11, 2021. Patients with liver enzyme levels higher than twice the upper limit of the reference range for aspartate aminotransferase(AST) and/or alanine aminotransferase (ALT) were included. Patients with unexplained pruritus and elevated fasting biliary acid (FBA) levels were considered ICP. The remaining cases were used as the control group. There were a total of 1751 patients in the study period. Among them, 126 had elevated liver enzymes. Nineteen of these cases had also ICP. AST and ALT values were statistically higher in the ICP group. Demographic features, clinical characteristics, and perinatal outcomes were similar between the groups. The rate of ICP in pregnant women with COVID-19 was similar to the literature in this study. Although the preterm delivery rates for both groups were higher than in the current literature, the preterm delivery rates in the study and control groups were similar. Elevated liver enzymes can be observed in pregnant women with COVID-19 with higher rates of preterm delivery compared to the previous literature. However, the diagnosis of ICP in addition to elevated liver enzymes seems to have no significant impact on the perinatal outcomes. Future studies conducted on larger populations are necessary to confirm these results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) was first reported in the Wuhan State of China in December 2019. In March 2020, World Health Organization declared the disease a pandemic and described it as coronavirus disease (COVID-19) [1]. As scientific knowledge on SARS-CoV-2 increases, gastrointestinal symptoms like nausea, vomiting, diarrhea, and abdominal pain have been observed in addition to lower respiratory system symptoms like fever, coughing, dyspnea, and myalgia [2, 3]. The virus is capable of involving numerous organs due to angiotensin-converting enzyme-2 (ACE-2) receptors found in various systems in the organism [2, 3]. Studies have demonstrated the presence of ACE-2 receptors in the liver, hepatic tracts, and gastrointestinal system; therefore, the virus may also affect these systems [4,5,6,7,8,9,10].
The liver serves as the primary center for the production of albumin, acute phase reactants, and coagulation factors. Liver function disorder accompanying acute respiratory syndrome (ARDS) may affect multiple system findings of COVID-19 such as coagulopathy and multiple organ failure. Also, the liver is the primary metabolical and detoxification organ for humans; therefore, even a moderate function loss changes the safety profiles and therapeutic effectiveness of the drugs metabolized in the liver [11]. Therefore, understanding the possible causes and clinical consequences of liver damage associated with COVID-19 may contribute to the creation of better clinical protocols.
Elevated liver enzymes were observed in approximately a quarter of COVID-19 cases [12]. Studies conducted on pregnant populations with COVID-19 reported similar results. Although the liver enzyme elevation levels were minor in most cases, the rate of high liver enzymes might reach above 40% in pregnant patients [13]. The rate of elevated liver enzymes was found in 4.6% of the cases in a previous study from our institution [14].
Although the etiology of intrahepatic cholestasis (ICP) is not completely understood, it is believed to be multifactorial. Genetic predisposition, hormonal factors, and environmental factors were suggested to be responsible. Increased estrogen levels due to pregnancy decrease the expressions of nuclear hepatic bile acid receptors and hepatic biliary canaliculi transport proteins in genetically susceptible women. Consequently, hepatic bile acid homeostasis gets disturbed and biliary acid levels increase [15]. The incidence of ICP in pregnant women varies between 0.2 and 2% [16].
The knowledge regarding the relationship between elevated liver enzymes and ICP in pregnant women with COVID-19 is still limited. Furthermore, the impact of these pathologies on perinatal outcomes in this specific population has not been clarified yet. This study aims to investigate the perinatal outcomes in COVID-19 pregnant women with intrahepatic cholestasis of pregnancy (ICP) and elevated liver enzymes.
Methods
Turkey Ministry of Health Ankara City Hospital is the main pandemic center in Turkey dealing with the most complicated cases. All pregnant women with COVID-19 are managed by an experienced multidisciplinary team according to the current guidelines [14].
This study is a retrospective cross-sectional study conducted on 1751 pregnant women with positive SARS-CoV-2 PCR test results between March 11, 2020, and August 11, 2021. Approvals from the Turkish Ministry of Health and local ethical commission (E2-21–1059) were obtained. Verification of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was made by a positive result of real-time polymerase chain reaction (RT-PCR) of nasopharyngeal and oropharyngeal samples [17].
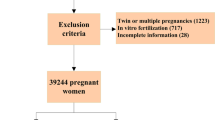
All of our patients were symptomatic after COVID-19. Patients with elevated liver enzymes or high FBA levels prior to SARS-CoV-2 positivity were not included in the study. Patients with levels of AST and/or ALT at least twice the upper limit of reference ranges were included in the study. Those with known active or chronic liver disease, hypertensive disease of pregnancy, and multiple pregnancies were excluded from the study. One hundred and twenty six patients were found to be meeting our inclusion criteria, and the elevation of liver enzymes to at least twice the upper limit rate was 7.1% (126/1751) for these patients. ICP diagnostic criteria according to guidelines: unexplained pruritus develops during pregnancy and abnormal liver function tests and/or bile acids are elevated, both of which resolve after delivery [18].On the other hand, hepatic enzyme elevations are seen in approximately 60% of ICP patients, while elevated FBA levels are seen in > 90% of patients [19]. In addition, some patients with SARS-CoV-2 liver injury may have elevated liver enzymes without ICP. Therefore, while planning our study, we considered itching together with increased FBA levels as ICP criteria. Fasting biliary acid (FBA) tests were performed on especially patients reporting nocturnal pruritus of hands and feet, and a result of ≥ 10 μmol/L was considered diagnostic (18). Of 31 patients whose FBA levels were tested, 12 had normal (< 10 μmol/L) and 19 had elevated (≥ 10 μmol/L) levels. Fifteen percent (19/126) of the patients with elevated liver enzyme levels were diagnosed with ICP. Cases with ICP along with elevated liver enzymes were used as the study group. Whereas, cases with solely elevated liver enzymes were served as the control group (Fig. 1). Demographic features, clinical characteristics and perinatal outcomes were compared between the groups.
Statistical analyzes were performed with the Statistical Package for the Social Sciences (SPSS.22, IBM SPSS Statistics for Windows, Version 22.0 Armonk, NY: IBM Corp.). Mean and standard deviation (SD) values were used for descriptive parameters that were normally distributed. The Mann–Whitney U test was conducted to compare the mean values of the parameters between the groups. Categorical variables were presented with numbers and percentages. The chi-square test was used to compare categorical variables. A two-tailed P value < 0.05 was regarded as statistically significant.
Results
Gravidity, parity, maternal age, living children, miscarriages, and body mass index (BMI) values were comparable between the groups as presented in Table 1.
Symptoms like fever, blood pressure, pulse rate, respiratory rate, and oxygen saturation were similar between the groups. While ALT and AST values were statistically higher in the ICP group, the other laboratory findings were similar between the groups. Although the length of hospital stay, gestational age at delivery, and APGAR scores were similar between the groups, gestational age at diagnosis was statistically higher in the ICP group (Table 2).
The most common symptoms such as coughing, dyspnea, and myalgia were similar for both groups. The rate of pruritus was significantly higher in the ICP group compared to the control group, while other symptoms have shown no statistically significant difference between the groups. While 71 patients were delivered during their hospital stays, 55 patients were still pregnant during the study period. Sixty one patients delivered in the control group and the preterm delivery rate was 43%. On the other hand,10 ICP patients were delivered during the study period and the preterm delivery rate was 50%. There was no significant difference between the groups in regard to the administration of antenatal corticosteroids therapy, systemic steroid therapy, oxygen support, maternal mortality, preterm delivery, admission to ICU, and admission to NICU (Table 3).
Discussion
The present study is, to the best of our knowledge, the first study in the literature that focuses on ICP patients with COVID-19 including the highest number of participants from a single pandemic center. SARS-CoV-2-related liver damage is described as the liver damage that occurred during the course and treatment of COVID-19 [10]. In SARS-CoV-2 patients, the overall incidence of elevated liver enzymes ranges between 2.5 and 76.3%. Furthermore, according to the results of a recent meta-analysis, AST and ALT values were outside the reference ranges for 20–22.5% and 14.6–20.1%, respectively [12]. Although COVID-19 related liver damage is usually reported to be mild in a significant number of patients, may be more adversely affected by those who have a severe disease course [12].
Liver damage has been reported in approximately 60% of patients with SARS, and positive SARS-CoV RT-PCR was detected in liver biopsy specimens of some SARS cases [20]. Similar to SARS-CoV, SARS-CoV-2 infiltrates the cells via ACE-2 receptors [2, 3]. ACE-2 is expressed in hepatocytes and biliary tract epithelial cells (cholangiocytes). An increase in ACE2 expressions is reported to be higher in cholangiocytes (59,7%) compared to hepatocytes (2.6%) [3]. Additionally, cholangiocytes play an important role in liver regeneration and immune response [21]. Thus, liver damage in COVID-19 patients may stem from not only hepatocyte damage but also from cholangiocyte damage [3, 21]. Autopsies performed on COVID-19 patient cadavers have shown irrefutable proof of secondary liver damage. Findings such as microvascular steatosis, hepatomegaly, permanent inflammation findings, and increased size of gall bladder are reported as histological findings that show the pathological changes caused by liver damage [22,23,24].
In most studies, higher levels of AST and ALT were regarded as the reflection of liver damage [3, 10, 11]. Some studies also made use of the parameters such as alkaline phosphatase (ALP), total bilirubin, gamma-glutamyltransferase (GGT), and albumin [25, 26]. The present study used the AST and ALT values, which are serum transaminases commonly used in general literature, as liver damage findings. In this study, rather than a minimal transferase elevation, we considered an elevation of twice the upper limit of the normal reference range in AST and/or ALT a significant increase. In addition, common comorbid diseases during pregnancy such as preeclampsia were excluded from the study. Consequently, our reported rate of patients with elevated levels of liver enzymes is lower when compared to the literature.
In studies including pregnant patients with COVID-19, similar results for liver damage were reported when compared to the non-pregnant population [27]. In pregnant patients with liver damage, worse course of COVID-19 and poor pregnancy outcomes were observed [28, 29]. The most important adverse pregnancy outcome is the increased rate of preterm delivery. According to the results of a current meta-analysis, the preterm delivery rate was reported to be 23% among pregnant women with COVID-19 [30]. Preterm delivery rate was reported to be more common among pregnant women with COVID-19 compared to the pregnant women with negative SARS-CoV-2 results [31]. To the best of our knowledge, there is no study in the literature investigating the rate of adverse pregnancy outcomes in SARS-CoV-2-positive pregnant women with elevated liver enzymes. Preterm delivery rates were higher both for the study and control groups in the present study. According to this data, we believe pregnant women with liver damage might be at greater risk of preterm delivery.
A mild course of COVID-19 and relatively favorable outcomes were reported for pregnant women with COVID-19 in the early months of the pandemic. However, recent studies indicated a worse course of COVID-19 and increased rates of complications among pregnant women with COVID-19. Especially the rates of mortality, admission to ICU, need for mechanical ventilation, and severe disease were higher in pregnant women with COVID-19 compared to the women with COVID-19 of similar ages [30, 32]. Although the two groups were similar with regard to adverse perinatal outcomes in the present study, higher rates of pregnancy complications and a worse course of the disease were observed for both groups compared to the findings of the previous studies including pregnant women with COVID-19.
Intrahepatic cholestasis of pregnancy is a multifactorial condition presented with normal/slightly elevated liver enzyme values [15, 16]. In the study group, both AST and ALT levels were significantly higher when compared to the group with only elevated liver enzymes. Considering the potential of increased poor perinatal outcomes women with ICP, early prediction of ICP among pregnant women with COVID-19 may help physicians to establish more effective management protocols.
Retrospective design and lack of information related to the delivery characteristics of some cases were the main limitations. Moreover, as FBA may be increased in patients with COVID-19, the diagnosis of ICP in the present study may not be optimal. Therefore, the readers may have concerns regarding the definite diagnosis of ICP. Furthermore, due to documentation restrictions, high number of emergency cesarean deliveries, and lack of consensus on the cesarean indications in this specific population, we could not present iatrogenic preterm delivery rates. On the other hand, the high number of participants and the assessment of the elevated liver enzymes’ impact on perinatal outcomes for the first time in the literature were the main strengths.
Conclusion
Elevated liver enzymes can be observed in pregnant women with COVID-19 with higher rates of preterm delivery compared to the previous literature. However, the diagnosis of ICP in addition to elevated liver enzymes seems to have no significant impact on the perinatal outcomes. Future studies conducted on larger populations are necessary to confirm these results.
Data Availability
The data supporting the analysis of this study can be obtained from the relevant author (R.DENİZLİ) upon request. Raw data is not publicly available due to institutional restrictions.
Code Availability
Not applicable.
References
-WHO, Coronavirus disease (COVID-19) dashboard. World Health Organization.2020. https://covid19.who.int/. Accessed 28 July 2021.
Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020;251(3):228–48. https://doi.org/10.1002/path.5471.
Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. BioRxiv. 2020. https://doi.org/10.1101/2020.02.03.931766.
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. China JAMA. 2020;323:1061–9. https://doi.org/10.1001/jama.2020.1585.
Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369: m1985. https://doi.org/10.1136/bmj.m1985.
Lin L, Jiang X, Zhang Z, Huang S, Zhang Z, Fang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997. https://doi.org/10.1136/gutjnl-2020-321013.
Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China JAMA Neurology. 2020;77:683–90. https://doi.org/10.1001/jamaneurol.2020.1127.
Kabbani N, Olds JL. Does COVID19 infect the brain? If so, smokers might be at a higher risk. Mol Pharmacol. 2020;97:351–3. https://doi.org/10.1124/molpharm.120.000014.
Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. https://doi.org/10.1038/s41368-020-0074-x.
Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41(1):20–32. https://doi.org/10.1111/liv.14730.
Bernal-Monterde V, Casas-Deza D, Letona-Giménez L, de la Llama-Celis N, Calmarza P, Sierra-Gabarda O, et al. SARS-CoV-2 Infection induces a dual response in liver function tests: association with mortality during hospitalization. Biomedicines. 2020;8(9):328. https://doi.org/10.3390/biomedicines8090328.
Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, et al. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52(4):584–99. https://doi.org/10.1111/apt.15916.
Chen L, Li Q, Zheng D, Jiang H, Wei Y, Zou L, et al. Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. N Engl J Med. 2020;382(25): e100. https://doi.org/10.1056/NEJMc2009226.
Sahin D, Tanacan A, Erol SA, YucelYetiskin FD, Besimoglu B, OzdenTokalioglu E, et al. Management of pregnant women with COVID-19: a tertiary pandemic center experience on 1416 cases. J Med Virol. 2021. https://doi.org/10.1002/jmv.27423.
Abu-Hayyeh S, Papacleovoulou G, Lövgren-Sandblom A, Tahir M, Oduwole O, Jamaludin NA, et al. Intrahepatic cholestasis of pregnancy levels of sulfated progesterone metabolites inhibit farnesoid X receptor resulting in a cholestatic phenotype. Hepatology. 2013;57(2):716–26. https://doi.org/10.1002/hep.26055.
Williamson C, Geenes V. Intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2014;124(1):120–33. https://doi.org/10.1097/AOG.0000000000000346.
Wang X, Tan L, Wang X, Liu W, Lu Y, Cheng L, et al. Comparison of nasopharyngeal and oropharyngeal swabs for SARS-CoV-2 detection in 353 patients received tests with both specimens simultaneously. Int J Infect Dis. 2020;94:107–9. https://doi.org/10.1016/j.ijid.2020.04.023.
- Royal College of Obstetricians and Gynaecologists (RCOG) (2011), Green-top guideline No 43: obstetric cholestasis. Oxford : RCOG Press. https://www.rcog.org.uk/media/neldxzix/gtg_43.pdf. Accessed 28 July 2022.
Bacq Y, Sapey T, Bréchot MC, Pierre F, Fignon A, Dubois F. Intrahepatic cholestasis of pregnancy: a French prospective study. Hepatology. 1997;26(2):358–64. https://doi.org/10.1002/hep.510260216.
Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, et al. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39(2):302–10. https://doi.org/10.1002/hep.20111.
Banales JM, Huebert RC, Karlsen T, Strazzabosco M, LaRusso NF, Gores GJ. Cholangiocyte pathobiology. Nat. Rev. Gastroenterol Hepatol. 2019;16:269–81. https://doi.org/10.1038/s41575-019-0125-y.
Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–2. https://doi.org/10.1016/S2213-2600(20)30076-X.
Liu Q, Wang RS, Qu GQ, Wang YY, Liu P, Zhu YZ, et al. Gross examination report of a COVID-19 death autopsy. Fa Yi Xue Za Zhi. 2020;36(1):21–3. https://doi.org/10.12116/j.issn.1004-5619.2020.01.005.
Wichmann D. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173(12):1030. https://doi.org/10.7326/L20-1206.
Labenz C, Toenges G, Wörns MA, Sprinzl MF, Galle PR, Schattenberg JM. Liver injury in patients with severe acute respiratory syndrome coronavirus-2 infection: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2021;33(9):1194–200. https://doi.org/10.1097/MEG.0000000000001827.
Du M, Yang DS, Liu M, Liu J. COVID-19 and liver dysfunction: epidemiology, association and potential mechanisms. Clin Res Hepatol Gastroenterol. 2021;21: 101793. https://doi.org/10.1016/j.clinre.2021.101793.
Deng G, Zeng F, Zhang L, Chen H, Chen X, Yin M. Characteristics of pregnant patients with COVID-19 and liver injury. J Hepatol. 2020;73(4):989–91. https://doi.org/10.1016/j.jhep.2020.06.022.
Antoun L, Taweel NE, Ahmed I, Patni S, Honest H. Maternal COVID-19 infection, clinical characteristics, pregnancy, and neonatal outcome: a prospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2020;252:559–62. https://doi.org/10.1016/j.ejogrb.2020.07.008.
Bellos I, Pandita A, Panza R. Maternal and perinatal outcomes in pregnant women infected by SARS-CoV-2: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:194–204. https://doi.org/10.1016/j.ejogrb.2020.11.038.
Khalil A, Kalafat E, Benlioglu C, O’Brien P, Morris E, Draycott T, et al. SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinicalMedicine. 2020;25: 100446. https://doi.org/10.1016/j.eclinm.2020.100446.
Di Toro F, Gjoka M, Di Lorenzo G, De Santo D, De Seta F, Maso G, et al. Impact of COVID-19 on maternal and neonatal outcomes: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27(1):36–46. https://doi.org/10.1016/j.cmi.2020.10.007.
Capobianco G, Saderi L, Aliberti S, Mondoni M, Piana A, Dessole F, et al. COVID-19 in pregnant women: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;252:543–58. https://doi.org/10.1016/j.ejogrb.2020.07.006.
Author information
Authors and Affiliations
Contributions
All authors have read and approved the final manuscript. Corresponding author RD contributed significantly to the concept, design, and intellectual content of the study and manuscript. All authors contributed to the following sections of the manuscript and fulfilled the conditions of being an article author.
Concept: RD, DŞ, AT, ÖMT, SS, BS, NF, MEMP, ÖK.
Design: RD, AT, DŞ, ÖK.
Data collecting: MEMP, BS, NF.
Experiments and procedures; RD, DŞ, AT, ÖMT.
Writing of article: RD, AT, DŞ.
Corresponding author
Ethics declarations
Ethics Approval
Approvals from the Turkish Ministry of Health and local ethical commission (E2-21–1059) were obtained.
Consent to participate
Not applicable.
Consent for Publication
All authors agree to publish the article.
Conflicts of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on COVİD-19.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Denızlı, R., Sakcak, B., Farisoğulları, N. et al. The İmpact of Elevated Liver Enzymes and İntrahepatic Cholestasis of Pregnancy on the Course of COVID-19 in Pregnant Women. SN Compr. Clin. Med. 4, 184 (2022). https://doi.org/10.1007/s42399-022-01267-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s42399-022-01267-1