Abstract
In the era of the COVID-19 pandemic declared in March 2020, widespread vaccination protocols were initiated to mitigate the severity and spread of COVID-19. Although COVID-19 vaccines have been generally considered safe, adverse events post-vaccination have been reported, including the development of demyelinating disease. We report a rare case of de novo aquaporin-4-positive neuromyelitis optica spectrum disorder (NMOSD) in an 80-year-old man following BNT162b SARS-CoV-2 vaccination to raise the awareness of this possible severe adverse event in an older adult. An 80-year-old South Asian man presented 2 days following his second dose of the Pfizer-BioNTech COVID-19 mRNA BNT162b2 vaccine with progressive left-sided leg weakness and numbness resulting in falls. MRI of the spine revealed a longitudinally extensive transverse myelitis from T3–T4 to T9–T10. Serum antibody testing revealed positive aquaporin-4 (AQP4) antibodies. He was diagnosed with AQP4-positive NMOSD and was treated with high-dose intravenous methylprednisolone and plasma exchange with some improvement. He was subsequently treated with mycophenolate mofetil and a slow steroid wean. This case report adds to the existing literature and suggests that COVID-19 vaccinations may trigger de novo NMOSD or NMOSD relapses in some individuals. Although rare, our patient presented with new-onset NMOSD in his 80 s following COVID-19 vaccination. As such, it is relevant to consider AQP4 testing in those presenting with a post-vaccination myelitis, regardless of age. Ongoing vaccine surveillance and research are needed to understand the risk of NMOSD post-COVID-19 vaccinations further.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
In the setting of the COVID-19 pandemic declared in March 2020, widespread vaccination protocols have been initiated to mitigate the severity and limit the spread of the COVID-19 virus. Although generally recommended, the safety and efficacy of COVID-19 vaccines are still under investigation. As with other vaccinations, there exists a risk of adverse events, including new-onset post-vaccination demyelinating disease. Although rare, we report the case of an 80-year-old man with no previous history of neurological or inflammatory disease presenting with longitudinally extensive transverse myelitis (LETM), diagnosed as de novo AQP4-positive NMOSD following BNT162b SARS-CoV-2 vaccination. It is plausible that vaccination may have triggered disease activity in an individual with underlying susceptibility. While seronegative post-vaccination myelitis is often monophasic, a positive aquaporin-4 (AQP4) antibody test suggests NMOSD and an increased risk of future relapses, and therefore NMOSD is an important consideration in individuals presenting with post-vaccination myelitis.
Case Presentation
A South Asian man in his early 80 s with no prior history of neurological symptoms presented to our hospital with falls within days of receiving his second COVID-19 vaccine. He was previously independent with no baseline disability. He received the first dose of the COVID-19 mRNA BNT162b2 vaccine in the spring of 2021 without any complications and then received the second dose in the early summer of 2021. Within 2 days of receiving his second dose, he started falling and noticed gait instability, difficulty voiding urine, progressive left-sided leg weakness, and numbness. He presented to our hospital after the weakness and numbness worsened, leading to multiple falls. On examination, he was afebrile and vitally stable. The cranial nerve exam was normal. Tone was normal in all four limbs. Upper extremity power was full. There was bilateral leg weakness in a pyramidal distribution, worse on the left (4-/5) than the right (4/5). His reflexes were 2 + and symmetric. Plantar responses were extensor bilaterally. Sensation to pinprick, light touch, and temperature was reduced in the left leg with a sensory level at T10 on the same side. Vibration sensation was reduced to the knee on the right and to the hip on the left. He was found to be in urinary retention requiring catheterization.
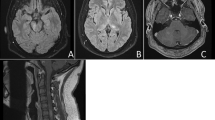
An MRI of the spine demonstrated a peripherally enhancing longitudinally extensive intramedullary lesion extending from T3–T4 down to T9–T10. Smaller, more chronic-appearing non-enhancing dorsal cord lesions were noted at C4–C5 and T1 (Fig. 1). An MRI of the brain did not show any evidence of intracranial demyelination. Serum aquaporin-4 (AQP4) antibodies and myelin oligodendrocyte glycoprotein (MOG) antibodies were both positive. C-reactive protein was mildly elevated at 10.9 mg/L. Serological screening for rheumatological and infectious diseases was unremarkable. Cerebrospinal fluid (CSF) analysis revealed a white blood cell count of 39 with 93% lymphocytes. Protein, glucose, cytology, and infectious studies were unremarkable. The oligoclonal band assay was negative for CSF-specific bands. Computer tomography (CT) of the chest, abdomen, and pelvis did not demonstrate any evidence of an underlying malignancy or infection.
MRI of longitudinal spinal cord lesions: T2-weighted sagittal view of the cervical and upper thoracic cord showing a longitudinally extensive cord signal abnormality (A, red arrows), predominantly involving the central aspect (B, red arrowhead) of the cord from T3–T4 down to T9–T10. T1-weighted sagittal view with gadolinium showing a peripheral enhancement pattern (C, green arrows). 338 × 160 mm (125 × 125 DPI)
In light of the above investigations, he was diagnosed with seropositive NMOSD and was treated accordingly with a 3-day course of high-dose (1 g) intravenous methylprednisolone. He experienced a mild improvement in his urinary dysfunction and left leg weakness. He then underwent five sessions of plasma exchange (PLEX). His lower extremity power improved to 5/5 on the right and 4 + /5 on the left following PLEX. He was maintained on prednisone 40 mg daily and started on mycophenolate mofetil with a slow steroid wean.
Repeat antibody testing after 2 weeks and prior to PLEX revealed that AQP4 antibodies remained positive, but MOG antibodies were negative. Cell-based MOG testing was not done, and, in retrospect, the initial positive MOG antibodies were felt to be falsely positive. On the 3-month follow-up visit, he denied any new symptoms and reported further improvement.
Discussion and Conclusions
This case illustrates AQP4 antibody-positive NMOSD in an 80-year-old man following his second dose of the Pfizer-BioNTech COVID-19 mRNA BNT162b2 vaccine. Brighton collaboration criteria supported a vaccine-related myelitis diagnosis (Level 2) [1]. A diagnosis of NMOSD was made based on the 2015 International Panel for Neuromyelitis Optica (NMO) Diagnosis criteria [2]. NMOSD is an antibody-mediated disease of the central nervous system. Although the average age of NMOSD onset is in the 3–4th decades, cases have been described in older individuals [3, 4]. An older age at onset is associated with earlier death due to myelitis and infection and in contrast to multiple sclerosis, relapse activity in NMOSD does not decrease with age [3, 4]. Typical presentations of NMOSD include attacks of severe unilateral, bilateral, or rapidly sequential optic neuritis and transverse myelitis, which generally involve three or more vertebral segments on MRI (termed longitudinally extensive transverse myelitis or LETM). In addition, other areas of the central nervous system (CNS) can also be affected, resulting in area postrema, other brainstem, diencephalic, or cerebral presentations in some patients [2].
Factors responsible for triggering CNS inflammatory diseases, including NMOSD, are not well understood, but evidence suggests immunizations may be implicated in some cases. In a study by Karussis and Petrou [5], vaccinations were estimated to carry an overall risk of 0.1% in triggering central nervous system (CNS) inflammatory diseases. The most common post-vaccination CNS syndromes in this study were acute optic neuritis and transverse myelitis. NMOSD, acute disseminated encephalomyelitis (ADEM), and encephalitis with white matter involvement were also reported [5]. Vaccinations that have been associated with NMOSD onset and/or relapses include influenza, tetanus and diphtheria, tetanus, diphtheria, and pertussis, human papillomavirus, pneumococcal, hepatitis A, hepatitis B, typhoid, yellow fever, and Japanese encephalitis vaccines [6]. Interestingly, the risk of an NMOSD relapse after vaccination seems to be most clearly observed in patients who are not on preventative immunotherapy [7].
The pathophysiology of vaccine-triggered CNS disease remains incompletely understood, but some studies suggest post-vaccination demyelination is most likely triggering clinical disease expression in individuals who already have an underlying disease process [8]. Evidence suggests that AQP4 antibodies may be detected long before clinical NMOSD onset, suggesting AQP4 antibody carriers can be asymptomatic for extended periods of time [9]. Theoretically, these individuals may be particularly susceptible to developing clinical symptoms when faced with a possible trigger.
In the era of the COVID-19 pandemic with increasing vaccination rates worldwide, vaccine safety remains at the forefront of discussion. Neurological symptoms which have been reported in the Centers for Disease Control Vaccine Adverse Event Reporting System include dizziness, headache, pain, muscle spasms, myalgia, and paresthesias as well as rare cases of tremor, diplopia, tinnitus, dysphonia, seizures, and reactivation of herpes zoster. There are also rare cases of stroke, Guillain–Barre syndrome, facial palsy, transverse myelitis, and acute disseminated encephalomyelitis [10]. With regards to NMOSD, there is very limited data linking COVID-19 vaccinations with disease onset. A case report from Fujikawa and colleagues [11] describes a 46-year-old woman presenting with LETM involving C6–T2 without enhancement diagnosed 10 days following the SARS-CoV-2 mRNA-1273 (Moderna) vaccine. In contrast to our case, serum AQP4 antibodies were negative. Another case report described a middle-aged woman who developed mild fever, diarrhea, and area postrema syndrome 3 days after her first dose of an “inactivated virus vaccine.” MRI brain demonstrated area postrema and bilateral hypothalamus lesions without gadolinium enhancement. Serum testing was positive for AQP4, antinuclear, SSA, SSB, Ro-52, and p-ANCA antibodies. The patient was diagnosed as AQP4-positive NMOSD with coexisting systemic autoimmunity [12].
Overall, the risk of CNS disease post-vaccination remains lower than rates following infections against which the vaccines are aimed to protect. Additionally, current epidemiological data suggests the benefits of vaccinations both at an individual and population level prevail over potential risks of CNS complications [5, 10]. We present the case of an 80-year-old man with no previous history of neurological or inflammatory disease presenting with LETM secondary to de novo AQP4-positive NMOSD following BNT162b SARS-CoV-2 vaccination. Although we cannot prove causality, it is plausible that vaccination may have triggered disease activity in an individual with underlying susceptibility. Thus, we believe that people with presumed post-vaccine myelitis should be tested for AQP4 antibodies even in older patients and, if the diagnostic criteria are met [2], managed like other patients with NMOSD to decrease the likelihood of relapses.
Data Availability
Not applicable.
Code Availability
All data and materials as well as software applications supporting our claims comply with field standards. No custom code has been used for this manuscript.
Abbreviations
- AQP4:
-
Aquaporin-4
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- CT:
-
Computer tomography
- LETM:
-
Longitudinally extensive transverse myelitis
- MOG:
-
Myelin oligodendrocyte glycoprotein
- NMOSD:
-
Neuromyelitis optica spectrum disorder
- PLEX:
-
Plasma exchange
References
Sejvar JJ, Kohl KS, Bilynsky R, Blumberg D, Cvetkovich T, Galama J, et al. Encephalitis, myelitis, and acute disseminated encephalomyelitis (ADEM): case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5771–92.
Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–89.
Collongues N, Marignier R, Jacob A, Leite M, Siva A, Paul F, et al. Characterization of neuromyelitis optica and neuromyelitis optica spectrum disorder patients with a late onset. Mult Scler J. 2013;20:1086–94.
Krumbholz M, Oy UH, Angstwurm K, Kleiter I, Jarius S, Paul F, et al. Very late-onset neuromyelitis optica spectrum disorder beyond the age of 75. J Neurol. 2015;262:1379–84.
Karussis D, Petrou P. The spectrum of post-vaccination inflammatory CNS demyelinating syndromes. Autoimmun Rev. 2014;13:215–24.
Kuhle. Plasma neurofilament light chain and glial fibrillary acidic protein levels are prognostic of disability worsening: a biosignature that helps differentiating active from non-active SPMS (2580) [Internet]. 2021. Available from: http://n.neurology.org/content/96/15_Supplement/2580.abstract. Accessed 18 Apr 2021
Mealy MA, Cook LJ, Pache F, Velez DL, Borisow N, Becker D, et al. Vaccines and the association with relapses in patients with neuromyelitis optica spectrum disorder. Mult Scler Relat Dis. 2018;23:78–82.
DeStefano F, Verstraeten T, Jackson LA, Okoro CA, Benson P, Black SB, et al. Vaccinations and risk of central nervous system demyelinating diseases in adults. Arch Neurol-chicago. 2003;60:504–9.
Nishiyama S, Ito T, Misu T, Takahashi T, Kikuchi A, Suzuki N, et al. A case of NMO seropositive for aquaporin-4 antibody more than 10 years before onset. Neurology. 2009;72:1960–1.
Goss AL, Samudralwar RD, Das RR, Nath A. ANA Investigates: neurological complications of COVID-19 vaccines. Ann Neurol. 2021;89:856–7.
Fujikawa P, Shah FA, Braford M, Patel K, Madey J. Neuromyelitis optica in a healthy female after severe acute respiratory syndrome coronavirus 2 mRNA-1273 vaccine. Cureus. 2021;13: e17961.
Chen S, Fan X-R, He S, Zhang J-W, Li S-J. Watch out for neuromyelitis optica spectrum disorder after inactivated virus vaccination for COVID-19. Neurol Sci. 2021;42:3537–9.
Author information
Authors and Affiliations
Contributions
• SK drafted the manuscript.
• GS and RS revised the manuscript.
• All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The need for approval was waived for a single case report.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Covid-19
Rights and permissions
About this article
Cite this article
Kuntz, S., Saab, G. & Schneider, R. Antibody-Positive Neuromyelitis Optica Spectrum Disorder After Second COVID-19 Vaccination: a Case Report. SN Compr. Clin. Med. 4, 130 (2022). https://doi.org/10.1007/s42399-022-01213-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s42399-022-01213-1