Abstract
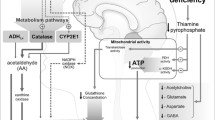
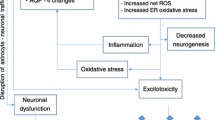
Rare but severe central nervous system complications associated with vitamin B deficiency, such as Wernicke encephalopathy, Korsakoff syndrome, and Marchiafava–Bignami disease, have been reported as a result of neurotoxicity related to alcohol use. Their pathophysiologic mechanisms remain unclear, particularly for Marchiafava–Bignami disease. These conditions remain underdiagnosed due to the non-specificity of their presenting symptoms and a lack of specific tests or diagnostic criteria. From a prognostic perspective, it is beneficial to start vitamin B complex supplementation before a definitive diagnosis. The mechanisms of thiamine deficiency–induced cell damage in Wernicke encephalopathy have been elucidated but remain unclear in Marchiafava–Bignami disease. We hypothesize that the mechanisms of Wernicke encephalopathy and Marchiafava–Bignami disease are different. Cytokine-mediated cytotoxic edema is a pathophysiological mechanism in Marchiafava–Bignami disease, in addition to vitamin B1 deficiency–related mechanisms. Although the amnesia of Korsakoff syndrome has generally been considered irreversible, recent studies have shown an improvement of cognitive function after memory rehabilitation. Alcohol-related central nervous system disorders associated with vitamin B deficiency occur in alcoholics. Rapid diagnosis and treatment of Wernicke encephalopathy and Marchiafava–Bignami disease have a profound effect on patient prognosis and prevent progression to Korsakoff syndrome. The newly proposed pathophysiological hypothesis of Marchiafava–Bignami disease may be useful for disease management in the future.




Similar content being viewed by others
Data Availability
The data used and analyzed during the current article are available from the corresponding author on reasonable request.
Abbreviations
- WE:
-
Wernicke encephalopathy
- KS:
-
Korsakoff syndrome
- MBD:
-
Marchiafava–Bignami disease
- GABA:
-
gamma-aminobutyric acid
- ATP:
-
adenosine triphosphate
- NADPH:
-
nicotinamide adenine dinucleotide phosphate
- NMDA:
-
N-methyl-d-aspartate
- GDF15:
-
growth differentiation factor 15
- MRI:
-
magnetic resonance imaging
- FLAIR:
-
fluid-attenuated inversion recovery
- RCT:
-
randomized controlled trial
- DWI:
-
diffusion-weighted imaging
- ADC:
-
apparent diffusion coefficient
- AQP4:
-
aquaporin 4
- AMPA:
-
α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
References
Keil VC, Greschus S, Schneider C, Hadizadeh DR, Schild HH. The whole spectrum of alcohol-related changes in the CNS: practical MR and CT imaging guidelines for daily clinical use. Rofo. 2015;187(12):1073–83.
Sechi G, Serra A. Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007;6(5):442–55.
Chandrakumar A, Bhardwaj A, ’t Jong GW. Review of thiamine deficiency disorders: Wernicke encephalopathy and Korsakoff psychosis. J Basic Clin Physiol Pharmacol. 2018;30(2):153–62.
Lonsdale D. Thiamine and magnesium deficiencies: keys to disease. Med Hypotheses. 2015;84(2):129–34.
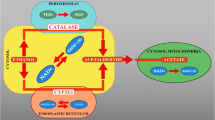
Alexander-Kaufman K, Harper C. Transketolase: observations in alcohol-related brain damage research. Int J Biochem Cell Biol. 2009;41(4):717–20.
Koulentaki M, Kouroumalis E. GABA(A) receptor polymorphisms in alcohol use disorder in the GWAS era. Psychopharmacology. 2018;235(6):1845–65.
Zeng WQ, Al-Yamani E, Acierno JS Jr, Slaugenhaupt S, Gillis T, MacDonald ME, et al. Biotin-responsive basal ganglia disease maps to 2q36.3 and is due to mutations in SLC19A3. Am J Hum Genet. 2005;77(1):16–26.
Huang W, Qin J, Liu D, Wang Y, Shen X, Yang N, et al. Reduced thiamine binding is a novel mechanism for TPK deficiency disorder. Mol Gen Genomics. 2019;294(2):409–16.
Harrigan GG, Maguire G, Boros L. Metabolomics in alcohol research and drug development. Alcohol Res Health. 2008;31(1):26–35.
Coleman JP, Smith CJ. X Pharm: the comprehensive pharmacology reference. 2007:1–6. https://doi.org/10.1016/b978-008055232-3.60227-2.
Jiang F, Guo N, Dusting GJ. Modulation of nicotinamide adenine dinucleotide phosphate oxidase expression and function by 3′,4′-dihydroxyflavonol in phagocytic and vascular cells. J Pharmacol Exp Ther. 2008;324(1):261–9.
Desjardins P, Butterworth RF. Role of mitochondrial dysfunction and oxidative stress in the pathogenesis of selective neuronal loss in Wernicke’s encephalopathy. Mol Neurobiol. 2005;31(1–3):17–25.
Miyaue N, Yabe H, Nagai M. Serum growth differentiation factor 15 levels and clinical manifestations in patients with thiamine deficiency. Neurol Clin Neurosci. 2020;8(5):245–50. https://doi.org/10.1111/ncn3.12390.
Yatsuga S, Fujita Y, Ishii A, Fukumoto Y, Arahata H, Kakuma T, et al. Growth differentiation factor 15 as a useful biomarker for mitochondrial disorders. Ann Neurol. 2015;78(5):814–23.
Fujita Y, Taniguchi Y, Shinkai S, Tanaka M, Ito M. Secreted growth differentiation factor 15 as a potential biomarker for mitochondrial dysfunctions in aging and age-related disorders. Geriatr Gerontol Int. 2016;16(Suppl 1):17–29.
Sivolap YP. The current state of S. S. Korsakov's concept of alcoholic polyneuritic psychosis. Neurosci Behav Physiol. 2005;35(9):977–82.
Charness ME, DeLaPaz RL. Mamillary body atrophy in Wernicke’s encephalopathy: antemortem identification using magnetic resonance imaging. Ann Neurol. 1987;22(5):595–600.
Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff syndrome and related neurologic disorders due to alcoholism and malnutrition. 2nd ed. Philadelphia: FA Davis; 1989. https://doi.org/10.1192/S0007125000006243.
Thomson AD, Cook CC, Touquet R, Henry JA, Royal College of Physicians, London. The Royal College of Physicians report on alcohol: guidelines for managing Wernicke’s encephalopathy in the accident and Emergency Department. Alcohol Alcohol. 2002;37(6):513–21.
Harper CG, Giles M, Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986;49(4):341–5.
Caine D, Halliday GM, Kril JJ, Harper CG. Operational criteria for the classification of chronic alcoholics: identification of Wernicke’s encephalopathy. J Neurol Neurosurg Psychiatry. 1997;62(1):51–60.
Galvin R, Bråthen G, Ivashynka A, Hillbom M, Tanasescu R, Leone MA, et al. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17(12):1408–18.
Liou KC, Kuo SF, Chen LA. Wernicke encephalopathy with atypical magnetic resonance imaging. Am J Emerg Med. 2012;30(9):2086.e1–3.
Zuccoli G, Gallucci M, Capellades J, Regnicolo L, Tumiati B, Giadás TC, et al. Wernicke encephalopathy: MR findings at clinical presentation in twenty-six alcoholic and nonalcoholic patients. AJNR Am J Neuroradiol. 2007;28(7):1328–31.
Sinha S, Kataria A, Kolla BP, Thusius N, Loukianova LL. Wernicke encephalopathy-clinical pearls. Mayo Clin Proc. 2019;94(6):1065–72.
Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44(9):911–5.
Tallaksen CM, Sande A, Bøhmer T, Bell H, Karlsen J. Kinetics of thiamin and thiamin phosphate esters in human blood, plasma and urine after 50 mg intravenously or orally. Eur J Clin Pharmacol. 1993;44(1):73–8.
Ambrose ML, Bowden SC, Whelan G. Thiamin treatment and working memory function of alcohol-dependent people: preliminary findings. Alcohol Clin Exp Res. 2001;25(1):112–6.
Day E, Bentham PW, Callaghan R, Kuruvilla T, George S. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;2013(7):CD004033. https://doi.org/10.1002/14651858.CD004033.pub3.
Blansjaar BA, Van Dijk JG. Korsakoff minus Wernicke syndrome. Alcohol Alcohol. 1992;27:435–7.
Mitchell AS, Chakraborty S. What does the mediodorsal thalamus do? Front Syst Neurosci. 2013;7:37.
Harding A, Halliday G, Caine D, Kril J. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain. 2000;123(Pt 1):141–54.
Fama R, Marsh L, Sullivan EV. Dissociation of remote and anterograde memory impairment and neural correlates in alcoholic Korsakoff syndrome. J Int Neuropsychol Soc. 2004;10(3):427–41.
Kopelman MD, Thomson AD, Guerrini I, Marshall EJ. The Korsakoff syndrome: clinical aspects, psychology and treatment. Alcohol Alcohol. 2009;44(2):148–54.
Van Oort R, Kessels RP. Executive dysfunction in Korsakoff’s syndrome: time to revise the DSM criteria for alcohol-induced persisting amnestic disorder? Int J Psychiatry Clin Pract. 2009;13(1):78–81.
Brion M, Pitel AL, Beaunieux H, Maurage P. Revisiting the continuum hypothesis: toward an in-depth exploration of executive functions in Korsakoff syndrome. Front Hum Neurosci. 2014;8:498.
Pitel AL, Chételat G, Le Berre AP, Desgranges B, Eustache F, Beaunieux H. Macrostructural abnormalities in Korsakoff syndrome compared with uncomplicated alcoholism. Neurology. 2012;78(17):1330–3.
Covell T, Siddiqui W. Korsakoff Syndrome. 2019 May 5. StatPearls [Internet]. [cited 2020 May 16]. Treasure Island (FL): StatPearls Publishing; 2020. Available from http://www.ncbi.nlm.nih.gov/books/NBK539854/
Brokate B, Hildebrandt H, Eling P, Fichtner H, Runge K, Timm C. Frontal lobe dysfunctions in Korsakoff’s syndrome and chronic alcoholism: continuity or discontinuity? Neuropsychology. 2003;17(3):420–8.
Ridley NJ, Draper B, Withall A. Alcohol-related dementia: an update of the evidence. Alzheimers Res Ther. 2013;5(1):3. https://doi.org/10.1186/alzrt157 eCollection 2013.
Arts NJ, Walvoort SJ, Kessels RP. Korsakoff’s syndrome: a critical review. Neuropsychiatr Dis Treat. 2017;13:2875–90.
Svanberg J, Evans JJ. Neuropsychological rehabilitation in alcohol-related brain damage: a systematic review. Alcohol Alcohol. 2013;48(6):704–11.
Arbelaez A, Pajon A, Castillo M. Acute Marchiafava-Bignami disease: MR findings in two patients. AJNR Am J Neuroradiol. 2003;24(10):1955–7.
Tetsuka S, Kamimura T, Ohki G, Hashimoto R. Reversible lesion in the splenium of the corpus callosum in a patient with chronic alcoholism. J Gen Fam Med. 2020;21(3). https://doi.org/10.1002/jgf2.308.
Fernandes LMP, Bezerra FR, Monteiro MC, Silva ML, de Oliveira FR, Lima RR, et al. Thiamine deficiency, oxidative metabolic pathways and ethanol-induced neurotoxicity: how poor nutrition contributes to the alcoholic syndrome, as Marchiafava-Bignami disease. Eur J Clin Nutr. 2017;71(5):580–6.
Tetsuka S. Reversible lesion in the splenium of the corpus callosum. Brain Behav. 2019;9(11):e01440.
Oster J, Doherty C, Grant PE, Simon M, Cole AJ. Diffusion-weighted imaging abnormalities in the splenium after seizures. Epilepsia. 2003;44(6):852–4.
Galnares-Olalde JA, Vázquez-Mézquita AJ, Gómez-Garza G, Reyes-Vázquez D, Higuera-Ortiz V, Alegría-Loyola MA, et al. Cytotoxic lesions of the corpus callosum caused by thermogenic dietary supplements. AJNR Am J Neuroradiol. 2019;40(8).
Menezes RR, Godin AM, Rodrigues FF, Coura GME, Melo ISF, Brito AMS, et al. Thiamine and riboflavin inhibit production of cytokines and increase the anti-inflammatory activity of a corticosteroid in a chronic model of inflammation induced by complete Freund's adjuvant. Pharmacol Rep. 2017;69(5):1036–43.
Bjørkhaug ST, Neupane SP, Bramness JG, Aanes H, Skar V, Medhus AW, et al. Plasma cytokine levels in patients with chronic alcohol overconsumption: relations to gut microbiota markers and clinical correlates. Alcohol. 2019.
Pradier B, Erxlebe E, Markert A, Rácz I. Microglial IL-1β progressively increases with duration of alcohol consumption. Naunyn Schmiedeberg's Arch Pharmacol. 2018;391(4):455–61.
Kakou M, Velut S, Destrieux C. Arterial and venous vascularization of the corpus callosum. Neurochirurgie. 1998;44(1 Suppl):31–7.
Kahilogullari G, Comert A, Ozdemir M, Brohi RA, Ozgural O, Esmer AF, et al. Arterial vascularization patterns of the splenium: an anatomical study. Clin Anat. 2013;26(6):675–81.
Heinrich A, Runge U, Khaw AV. Clinicoradiologic subtypes of Marchiafava-Bignami disease. J Neurol. 2004;251(9):1050–9.
Rosa A, Demiati M, Cartz L, Mizon JP. Marchiafava-Bignami disease, syndrome of interhemispheric disconnection, and right-handed agraphia in a left-hander. Arch Neurol. 1991;48(9):986–8.
Hillbom M, Saloheimo P, Fujioka S, Wszolek ZK, Juvela S, Leone MA. Diagnosis and management of Marchiafava-Bignami disease: a review of CT/MRI confirmed cases. J Neurol Neurosurg Psychiatry. 2014;85(2):168–73.
Dong X, Bai C, Nao J. Clinical and radiological features of Marchiafava-Bignami disease. Medicine (Baltimore). 2018;97(5):e9626.
Acknowledgments
The authors would like to thank Enago (www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this work.
Corresponding author
Ethics declarations
Ethics Approval
This manuscript is a review article and does not contain any studies with human participants or animals performed by any of the authors and has included unidentifiable information. An informed consent was waived.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Medicine
Rights and permissions
About this article
Cite this article
Tetsuka, S., Hashimoto, R. Alcohol-Related Central Nervous System Disorders Associated with Vitamin B Deficiency. SN Compr. Clin. Med. 3, 528–537 (2021). https://doi.org/10.1007/s42399-021-00741-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42399-021-00741-6