Abstract
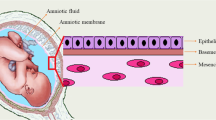
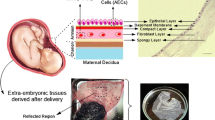
This review focuses on the probable anti-cancer mechanisms of human amniotic membrane (AM) that may be very helpful for ongoing cancer research activities with AM. A thorough search was conducted on PubMed for any published literature on the anti-cancer role of human AM using the key words, e.g., AM, function of AM, angiogenesis prevention, apoptosis induction by AM. No particular exclusion criteria were set. We selected resources from 1960 to 2018 with special focus on articles published during the last 7 years that revealed information regarding AM-derived factors and their specific functions to prevent cancer. Many studies suggest that human AM-derived epithelial stem cells (AM-hAECs) and mesenchymal stem cells (AM-hMSCs) secrete various factors, e.g., thrombospondin (TSP), tissue inhibitor metalloproteinase (TIMP), plasminogen activator inhibitors (PAI), IL-1 receptor antagonist (IL1RN), granulocyte monocyte-colony stimulating factor (GM-CSF), cytokines specially IL-6 and IL-10, various essential markers, and proteins which most predominantly increase the AM’s anti-cancer activity. This work gives an overview of the latest findings on AM function and evaluates its potential use in cancer treatment. Though various researches are being performed now on the anti-neoplastic properties of AM, the mechanism of these effects is not clear yet. Therefore, it has a great demand to unveil the mood of action of AM as to exert anti-cancer activity. From the meta-analysis of previous data, this review has pointed out an anti-cancer mechanism of AM that would help to use it as an anti-cancer therapy.



Similar content being viewed by others
Abbreviations
- AM :
-
amniotic membrane
- WHO :
-
World Health Organization
- AM-hAECs :
-
AM-derived epithelial stem cells
- AM-hMSCs :
-
AM-derived mesenchymal stem cells
- TSP :
-
thrombospondin
- TIMP :
-
tissue inhibitor metalloproteinases
- PAI :
-
plasminogen activator inhibitors
- IL1RN :
-
IL-1 receptor antagonist
- GM-CSF :
-
granulocyte monocyte-colony stimulating factor
- IL :
-
interleukin
- HLA :
-
human leukocyte antigen
- SLPI :
-
secretory leukocyte protease inhibitor
- HGF :
-
hepatocyte growth factor
- TGF :
-
tumor growth factor
- bFGF :
-
basic fibroblast growth factor
- KD :
-
kilo dalton
- MMP :
-
matrix metalloproteinase
- G0/G1 :
-
Gap0/Gap1
- S Phase :
-
synthesis phase
- IFN :
-
interferon
- CDK :
-
cyclin-dependent kinases
- MCM :
-
minichromosome maintenance complex
- PCNA :
-
proliferating cell nuclear antigen
- CDKN :
-
CDK inhibitor
- CUL1 :
-
Cullin-1
- pRB :
-
retinoblastoma protein
- HSP :
-
heat shock protein
- NF-kb :
-
nuclear factor kappa B
- APAF-1 :
-
apoptotic protease activating factor-1.
References
Bray F, Ferlay J, Soerjomataram I. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Damyanov CA, Maslev IK, Pavlov VS, Avramov L. Conventional treatment of cancer realities and problems. Annals of Comp and Alt Med. 2018;1(1):1–9.
Krishnamoorthy M, Prabhu A. Anticancer activitiy of Cynodon dactylon L . extract on ehrlich ascites carcinoma. J Environ Res Develop. 2011;5:551–7.
Chatterjee D, Sahu RK, Jha AK, Dwivedi J. Evaluation of antitumor activity of Cuscuta Reflexa Roxb ( Cuscutaceae ) against Ehrlich ascites carcinoma in Swiss albino mice. Trop J Pharm Res. 2011:447–54.
Sagar J, Chaib B, Sales K, Winslet M, Seifalian A. Role of stem cells in cancer therapy and cancer stem cells : a review. Cancer Cell Int. 2007;11:1–11.
Mamede AC, Guerra S, Laranjo M, Carvalho MJ, Oliveira RC, Gonçalves AC, et al. Selective cytotoxicity and cell death induced by human amniotic membrane in hepatocellular carcinoma. Med Oncol. 2015;32(12):257.
Ahmed AU, Alexiades NG, Lesniak MS. The use of neural stem cells in cancer gene therapy: predicting the path to the clinic. Curr Opin Mol Ther. 2011;12:546–52.
Moreau P, Avet-loiseau H, Harousseau J, Attal M. Current trends in autologous stem-cell transplantation for myeloma in the era of novel therapies. J Clin Oncol. 2011;29:1898–906.
Niknejad H, Khayat-khoei M, Peirovi H. Human amniotic epithelial cells induce apoptosis of cancer cells : a new anti-tumor therapeutic strategy. J Cytotherapy. 2014;16:33–40.
Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian M, et al. Properties of the amniotic membrane for potential use in tissue. Eur Cell Mater. 2008;15:88–99.
Seo JH, Kim YH, Kim JS. Properties of the amniotic membrane may be applicable in cancer therapy. Med Hypotheses. 2008;70:812–4.
Lacout A, Marcy P. Inhibition of MMPs might increase anticancer properties of amniotic epithelial cells. Med Hypotheses. 2012;78:690–1.
Jiao H, Guan F, Yang B. Human amniotic membrane derived-mesenchymal stem cells induce C6 glioma apoptosis in vivo through the Bcl-2 / caspase pathways. Mol Biol Rep. 2012;39:467–73.
Magatti M, De MS, Vertua E, Parolini O. Amniotic membrane-derived cells inhibit proliferation of cancer cell lines by inducing cell cycle arrest. J Cell Mol Med. 2012;16:2208–18.
Pigeon J, River B. Treatment of second-degree burns with amniotic membranes. Can Med Assoc J. 1960;83:844–5.
Malhotra C, Jain AK. Human amniotic membrane transplantation: different modalities of its use in ophthalmology. World J Transplant. 2014;4:111–21.
Agarwal A, Shankar S, Singh G, et al. Pleiotropic properties of amniotic membrane for modulation of periodontal healing. Int J Dent Med Res. 2014;1:110–7.
Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring HJ, Evangelista M, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells. 2008;26:300–11.
Ilancheran S, Moodley Y, Manuelpillai U. Human fetal membranes: a source of stem cells for tissue regeneration and repair? Placenta. 2009;30:2–10.
Subrahmanyam M. Amniotic membrane as a cover for microskin grafts. Br J Plast Surg. 1995;48:477–8.
Halim AS, Khoo TL, Yussof SJM. Biologic and synthetic skin substitutes: an overview. Indian J PlastSurg. 2010;43:23–8.
Trelford J, Trelford-Sauder M. The amnion in surgery, past and present. Am J Obstet Gynecol. 1979;134:833–45.
Hossain ML, Islam MM, Diba F, et al. The synergistic effect of AM and MO derived gel in burn and wound healing. Int J Complement Alt Med. 2018;11:21–6.
Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2:1003–5.
Ravishanker R, Bath AS, Roy R. “ Amnion Bank ”— the use of long term glycerol preserved amniotic membranes in the management of superficial and superficial partial thickness burns. Burns. 2003;29:369–74.
Aziz NA, Nazly H, Norimah Y Human amniotic membrane: basic science and clinical application. 1st ed. World Scientific; 2017.
Sharma SC, Bagree MM, Bhat AL, Banga BB, Singh MP. Amniotic membrane is an effective burn dressing material. Jpn J Surg. 1985;1(15):140–3.
Bose B. Burn wound dressing with human amniotic membrane. Ann R Coll Surg. 1979;61:444.
Rocha SC, Baptista CJ. Biochemical properties of amniotic membrane. V: Mamede AC, Botelho MF, ur. Amniotic membrane. Netherlands: Springer; 2015;19–40.
Kirschbaum S, Hernandez H. Use of amnion in extensive burns. In: 3rd International Congress in Plastic Surgery. Amsterdam: Excerpta Medica; 1963. p. 152–62.
Burgos H, Sergeant R. Lyophilized human amniotic membranes used in reconstruction of the ear. J R Soc Med. 1983;76:433.
Saarela J, Ylikarpp R, Rehn M, Purmonen S. Comptete primary structure of two variant forms of human type XVIII collagen and tissue-specific differences in the expression of the corresponding transcripts. Matrix Biol. 1998;16:319–28.
Li X, Fu G, Fan Y, Shi C, Liu X, Xu G, et al. Potent inhibition of angiogenesis and liver tumor growth by administration of an aerosol containing a transferrin-liposome- endostatin complex. World J Gastroenterol. 2003;9:262–6.
Folkman J. Antiangiogenesis in cancer therapy—endostatin and its mechanisms of action. Exp Cell Res. 2006;312:594–607.
Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med. 2002;6:1–12.
Hao Y, Ma DH, Hwang DG, Kim W, Ph D, Zhang F, et al. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000;19:348–52.
Walker AB, Cooney DR, Allen JE. Use of fresh amnion as burn dressing. J Pediatr Surg. 1977;12:391–5.
Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases : evolution , structure and function. Biochim Biophys Acta. 2000;1477:267–83.
Jennifer CCand Frank CC. Obesity and breast cancer: the roles of peroxisome proliferator-activated receptor-γ and plasminogen activator inhibitor-1. PPAR Res 2009: 345320.
Rerolle JP, Rehertig A, Nguyen G, Srayer JDAnd Rondeau EP. Plasminogen activator inhibitor type 1 is a potential target in renal fibrogenesis. Kidney Int. 2000;58:1841–50.
Matsuo Y, Sawai H, Xu D, Ochi N, Yasuda A, Takahashi H, et al. IL-1 a secreted by colon cancer cells enhances angiogenesis : the relationship between IL-1 a release and tumor cells ’ potential for liver metastasis. J Surg Oncol. 2009;99:361–7.
Matsuo Y, Sawai H, Ochi N, Yasuda A, Takahashi H, Funahashi H, et al. Interleukin-1alpha secreted by pancreatic cancer cells promotes angiogenesis and its therapeutic implications. J Surg Res. 2009;153:274–81.
Ma J, Sawai H, Matsuo Y, Ochi N, et al. Interleukin-1α enhances angiogenesis and is associated with liver metastatic potential in human gastric cancer cell lines. J Surg Res. 2008;204:197–204.
Mishra S, Singh S. Human amniotic membrane: can it be a ray of hope in periodontal regeneration? Indian J Dent Res. 2014;3:118–21.
La E, Rundhaug JE, Fischer SM. Role of intracellular interleukin-1 receptor antagonist in skin carcinogenesis. Molecular Carcinogenesis: Published in cooperation with the University of Texas MD Anderson Cancer Center 2001; 223:218–23.
Elaraj DM, Weinreich DM, Varghese S, Puhlmann M, Hewitt SM, Carroll NM, et al. The role of interleukin 1 in growth and metastasis of human cancer xenografts. Clin Cancer Res. 2006;12:1088–97.
Dickson MA, Cancer C, Published R, May O. Molecular pathways : CDK4 inhibitors for cancer therapy. Clin Cancer Res. 2014;20:3379–83.
Giuffrida D, Rogers IM, Nagy A, Calogero AE, Brown TJ, Casper RF. Human embryonic stem cells secrete soluble factors that inhibit cancer cell growth. Cell Prolif. 2009;42(6):788–98.
Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–66.
Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cipl is a potent inhibitor of G l cyclin-dependent kinases. Cell. 1993;75:805–16.
Guo W, Shang F, Liu Q, Urim L, West-mays J, Taylor A. Differential regulation of components of the ubiquitin-proteasome pathway during Lens cell differentiation. Invest Ophthalmol Vis Sci. 2004;45:1194–201.
Horne MC, Donaldson KL, Goolsby GL, Tran D, Mulheisen M, Hell JW, et al. Cyclin G2 is up-regulated during growth inhibition and B cell antigen receptor-mediated cell cycle arrest. J Biol Chem. 1997;272:12650–61.
Bennin DA, Don ASA, Brake T, Mckenzie JL, Ortiz L, Anna A, et al. Cyclin G2 associates with protein phosphatase 2A catalytic and regulatory B ‘ subunits in active complexes and induces nuclear aberrations and a G 1 / S phase cell cycle arrest. J Biol Chem. 2002;277:27449–67.
Martínez-gac L, Marque M, García Z, Campanero MR, Carrera AC. Control of cyclin G2 mRNA expression by forkhead transcription factors : novel mechanism for cell cycle control by phosphoinositide 3-kinase and forkhead. Mol Cell Biol. 2004;24:2181–9.
Wirt SE, Sage J. p107 in the public eye : an Rb understudy and more. Cell Div. 2010;5:9.
Workman P. Combinatorial attack on multistep oncogenesis by inhibiting the Hsp90 molecular chaperone. Cancer Lett. 2004;2010206:149–57.
Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Publ Group. 2010;10:537–49.
Pearl LH, Prodromou C, Workman P. The Hsp90 molecular chaperone : an open and shut case for treatment. Biochem J. 2008;410:439–53.
Eskew JD, Sadikot T, Morales P, Duren A, Dunwiddie I, Swink M, et al. Development and characterization of a novel C-terminal inhibitor of Hsp90 in androgen dependent and independent prostate cancer cells. BMC Cancer. 2011;11:468.
Sarkar S, Dutta D, Samanta SK, Bhattacharya K, Pal BC, Li J, et al. Oxidative inhibition of Hsp90 disrupts the super-chaperone complex and attenuates pancreatic adenocarcinoma in vitro and in vivo. Int J Cancer J Int du Cancer. 2013;132(3):695–706.
Graham B, Curry J, Smyth T, Fazal L, Feltell R, Harada I, et al. The heat shock protein 90 inhibitor, AT13387, displays a long duration of action in vitro and in vivo in non-small cell lung cancer. Cancer Sci. 2012;103(3):522–7.
Rajan A, Kelly RJ, Trepel JB, Kim YS, Alarcon SV, Kummar S, et al. A phase I study of PF-04929113 ( SNX-5422 ), an orally bioavailable heat shock protein 90 inhibitor , in patients with refractory solid tumor malignancies and lymphomas. Clin Cancer Res. 2011;17:6831–9.
Sequist LV, Gettinger S, Senzer NN, Martins RG, Ja PA, Gray JE, et al. Activity of IPI-504 , a novel heat-shock protein 90 inhibitor , in patients with molecularly defined non – small-cell lung cancer. J Clin Oncol. 2010;28:4953–60.
De Bono JS, Kristeleit R, Tolcher A, Fong P, Pacey S, Karavasilis V, et al. Phase I pharmacokinetic and pharmacodynamic study of LAQ824 , a hydroxamate histone deacetylase inhibitor with a heat shock protein-90 inhibitory profile , in patients with advanced solid tumors. Clin Cancer Res. 2008;14:6663–73.
Lancet JE, Gojo I, Burton M, Quinn M, Tighe SM, Kersey K, et al. Phase I study of the heat shock protein 90 inhibitor alvespimycin ( KOS-1022, 17-DMAG ) administered intravenously twice weekly to patients with acute myeloid leukemia. Leukemia. 2010;24:699–705.
Al SL, Walsby E, Gilkes A, Tonks A, Walsh V, Mills K. Heat shock protein 90 inhibition is cytotoxic to primary AML cells expressing mutant FLT3 and results in altered downstream signalling. Br J Haematol. 2008;141:483–93.
Lewis J, Devin A, Miller A, Lin Y, Rodriguez Y, Neckers L, et al. Disruption of hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-kappaB activation. J Biol Chem. 2000;275:10519–26.
Dias S, Shmelkov SV, Lam G, Rafii S, Dias S, Shmelkov SV, et al. VEGF 165 promotes survival of leukemic cells by Hsp90-mediated induction of Bcl-2 expression and apoptosis inhibition. Blood. 2002;99:2532–40.
Pandey P, Saleh A, Nakazawa A, Kumar S, Srinivasula SM, Kumar V, et al. Negative regulation of cytochrome c -mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J. 2000;19:4310–22.
Brunelle JK, Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci. 2009;122:437–41.
Zander T, Kraus A, Grommes C, Schlegel U, Feinstein D, Klockgether T, et al. Induction of apoptosis in human and rat glioma by agonists of the nuclear receptor PPARgamma. J Neurochem. 2002;81:1052–60.
Yin C, Knudson MC, Korsmeyer JS, Dyke VT. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature. 1997;385:637–40.
Williams GH, Stoeber K. The cell cycle and cancer. J Pathol. 2012;226:352–64.
Shao C, Sima J, Zhang SX, Jin J, Reinach P, Wang Z, et al. Suppression of corneal neovascularization by PEDF release from human amniotic membranes. Invest Ophthalmol Vis Sci. 2004;45:1758–62.
Mahgoub MA, Ammar A, Fayez M, Edris A, Hazem A, Akl M, et al. Neovascularization of the amniotic membrane as a biological immune barrier. Transplant Proc. 2004;36:1194–8.
Jiang A, Li C, Gao Y, et al. In vivo and in vitro inhibitory effect of amniotic extraction on neovascularization. Cornea. 2006;25:36–40.
Kobayashi N, Kabuyama Y, Sasaki S, Kato K, Homma Y. Suppression of corneal neovascularization by culture supernatant of human amniotic cells. Cornea. 2002;21:62–7.
Ma DH, Yao JY, Yeh LK, et al. In vitro antiangiogenic activity in ex vivo expanded human limbocorneal epithelial cells cultivated on human amniotic membrane. Invest Ophthalmol Vis Sci. 2004;45:2586–95.
Hori J, Wang M, Kamiya K, Takahashi H, Sakuragawa N. Immunological characteristics of amniotic epithelium. Cornea. 2006;25:53–8.
Li H, Niederkorn JY, Neelam S, Mayhew E, Word RA, McCulley JP, et al. Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:900–7.
Li W, He H, Kawakita T, Espana EM, Tseng SC. Amniotic membrane induces apoptosis of interferon-gamma activated macrophages in vitro. Exp Eye Res. 2006;82:282–92.
Zhou S, Chen J, Feng J. The effects of amniotic membrane on polymorphonuclear cells. Chin Med J. 2003;116:788–90.
Kim JC, Tseng SC. The effects on inhibition of corneal neovascularization after human amniotic membrane transplantation in severely damaged rabbit corneas. Korean J Ophthalmol. 995(9):32–46.
Kubo M, Sonoda Y, Muramatsu R, Usui M. Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci. 2001;42:1539–46.
Runic R, Lockwood CJ, LaChapelle L, et al. Apoptosis and Fas expression in human fetal membranes. J Clin Endocrinol Metab. 1998;83:660–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
Any of the authors did not use human or animal sample to perform the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Medicine
Rights and permissions
About this article
Cite this article
Hossain, L., Siddika, A., Adnan, M.H. et al. Human Amniotic Membrane and Its Anti-cancer Mechanism: a Good Hope for Cancer Therapy. SN Compr. Clin. Med. 1, 487–495 (2019). https://doi.org/10.1007/s42399-019-00090-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42399-019-00090-5