Abstract
Environmental degradation due to heavy metals has been studied worldwide due to their non-biodegradable and persistent nature. Among various heavy metals, chromium (VI) (Cr (VI)) and cadmium (Cd (II)) are toxic and enter into aquatic systems from anthropogenic activities. Therefore, considerable efforts have been made for the growth of a well-organized and cost-effective method for the removal of heavy metals. Among various methods, biosorption is cost-effective, environmentally friendly, and readily available. Biochar is porous, carboneous material produced from biomass by thermal decomposition under a limited supply of oxygen at a temperature below 900 ℃. The biomass comprises mainly of 35–50% cellulose, 20–35% hemicellulose, and 10–25% lignin, extractives, and ash. These components are accountable for most of the unique properties of the biochar. Further, the conversion of biomasses into biochar depends upon feedstock, particle size, temperature, reaction time, etc. The biomass with high carbon and lignin content produces biochar with high yield. Biochar has huge affinity for pollutants due to porous arrangement and functional groups such as carboxyl, hydroxyl, and phenolic. Therefore, biochars have been modified with different materials including nanoparticles to improve the removal capacity for pollutants. The sorption efficiency of biochar was found to be improved after modification with nanoparticles. Biochar-based nanocomposite has superior physical and chemical properties that arise due to a combination of advantages of both constituent particles. Thus, the fabrication of biochar based nanomaterials has been reported for the removal of Cd (II) and Cr (VI) from aqueous systems.
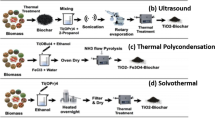
Graphical Abstract



Similar content being viewed by others
References
Abshire MK, Devor DE, Diwan BA, Shaughnessy JD, Waalkes MP (1996) In vitro exposure to cadmium in rat L6 myoblasts can result in both enhancement and suppression of malignant progression in vivo. Carcinogenesis 17:1349–1356
Agnieszka T, Zofia S, Patrycja B (2020) Biochar physicochemical properties: pyrolysis temperature and feedstock kind effects. Rev Environ Sci Biotechnol 19:191–215
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33
Ahmedna M, Marshall WE, Husseiny AA, Rao RM, Goktepe I (2004) The use of nutshell carbons in drinking water filters for removal of trace metals. Water Res 38:1062–1068
Akesson A, Bjellerup P, Lundh T, Lidfeldt J, Nerbrand C, Samsioe G (2006) Cadmium-induced effects on bone in a population-based study of women. Environ Health Perspect 114:830–834
Al-Wabel M, Al-Omran A, El-Naggar AH, Nadeem M, Usman ARA (2013) Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour Technol 131:374–379
Antal MJ, Gronli M (2003) The art, science, and technology of charcoal production. Ind Eng Chem Res 42(8):1619–1640
Bagreev A, Bandosz T, Locke D (2001) Pore structure and surface chemistry of adsorbents obtained by pyrolysis of sewage sludge-derived fertilizer. Carbon 39:1971–1979
Bala JD, Lalung J, Al-Gheethi AAS, Norli I (2016) A review on biofuel and bioresources for environmental applications. In: Ahmad M, Ismail M, Riffat S (eds) Renewable energy and sustainable technologies for building and environmental applications. Springer, Cham, pp 205–225
Baig SA, Zhu J, Muhammad N, Sheng T, Xu X (2014) Effect of synthesis methods on magnetic Kans grass biochar for enhanced As (III, V) adsorption from aqueous solutions. Biomass Bioenerg 71:299–310
Baselt RC, Cravey RH (1995) Disposition of toxic drugs and chemicals in man, 4th edn. Year Book Medical Publishers, Chicago, pp 105–107
Basu P (2013) Biomass gasification, pyrolysis and torrefaction practical design and theory, 2nd edn. Elsevier Inc., Amsterdam
Bridgwater AV (2012) Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 38:68–94
Cao XD, Ma LQ, Gao B, Harris W (2009) Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ Sci Technol 43:3285–3291
Carpenter D, Westover TL, Czernik S, Jablonski W (2014) Biomass feedstocks for renewable fuel production: a review of the impacts of feedstock and pretreatment on the yield and product distribution of fast pyrolysis bio-oils and vapors. Green Chem 16(2):384–406
Cui J, Zhang F, Li H, Cui J, RenY YuX (2020) Recent progress in biochar-based photocatalysts for wastewater treatment: synthesis, mechanisms, and applications. Appl Sci 10:1019–1032
Chen B, Chen Z, Lv S (2011) A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour Technol 102:716–723
Chen T, Zhang YX, Wang HT, Lu WJ, Zhou ZY, Zhang YC, Ren LL (2014) Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge. Bioresour Technol 164:47–54
Chun Y, Sheng G, Chiou CT, Xing B (2004) Compositions and sorptive properties of crop residue-derived chars. Environ Sci Technol 38:4649–4655
Cope CO, Webster DS, Sabatini DA (2014) Arsenate adsorption onto iron oxide amended rice husk char. Sci Total Environ 488:554–561
Costa M (1997) Toxicity and carcinogenicity of Cr(VI) in animal models and humans. Critical Rev Toxico 27:431–442
Demirbas A (2008) Heavy metal sorption onto agro-based waste materials: a review. J Hazard Mater 157:220–229
Devi P, Saroha AK (2014) Synthesis of the magnetic biochar composites for use as an adsorbent for the removal of pentachlorophenol from the effluent. Bioresour Technol 169:525–531
Dong HR, He Q, Zeng G, Tang L, Zhang C, Xie Y, Zeng Y, Zhao F, Wu Y (2016) Chromate removal by surface-modified nanoscale zero-valent iron: effect of different surface coatings and water chemistry. J Coll Inter Sci 471:7–13
Fang C, Zhang T, Li P, Jiang R, Wu S, Nie H, Wang Y (2015) Phosphorus recovery from biogas fermentation liquid by Ca-Mg loaded biochar. J Environ Sci 29:106–114
Fenglian Fu, Qi W (2011) Removal of heavy metal ions from wastewaters: A review. J Environ Manag 92:407–418
Fuertes A, Arbestain MC, Sevilla M, Macia-Agullo J, Fiol S, Lopez R, Smernik RJ, Aitkenhead W, Arce F, Macias F (2010) Chemical and structural properties of carbonaceous products obtained by pyrolysis and hydrothermal carbonisation of corn stover. Soil Res 48:618–626
Gan C, Liu YG, Tan XF, Wang S, Zeng G, Zheng B, Li T, Jiang Z, Liu W (2015) Effect of porous zinc–biochar nanocomposites on Cr (vi) adsorption from aqueous solution. RSC Adv 5:35107–35115
Goswami R, Shim J, Deka S, Kumari D, Kataki R, Kumar M (2016) Characterization of cadmium removal from aqueous solution by biochar produced from Ipomoea fistulosa at different pyrolytic temperatures. Ecol Eng 97:444–451
Hadjittofi L, Prodromou M, Pashalidis I (2014) Activated biochar derived from cactus fibres–preparation, characterization and application on Cu (II) removal from aqueous solutions. Bioresour Technol 159:460–464
Hamelink JL, Landrum PF, Harold BL, William BH (eds) (1994) Bioavailability: physical, chemical, and biological interactions. CRC Press Inc., Boca Raton
Hamid SBA, Chowdhury ZZ, Zain SM (2014) Base catalytic approach: a promising technique for the activation of biochar for equilibrium sorption studies of copper, Cu (II) ions in single solute system. Materials 7(4):2815–2832
Han Z, Sani B, Mrozik W, Obst M, Beckingham B, Karapanagioti HK, Werner D (2015) Magnetite impregnation effects on the sorbent properties of activated carbons and biochars. Water Res 70:394–403
He R, Peng Z, Lyu H, Huang H, Nan Q, Tang J (2017) Synthesis and characterization of an iron-impregnated biochar for aqueous arsenic removal. Sci Total Environ 612:1177–1186
Hossain M, Strezov V, Chan KY, Ziolkowski A, Nelson PF (2011) Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J Environ Manag 92:223–228
Hu X, Ding Z, Zimmerman AR, Wang S, Gao B (2015) Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res 68:206–216
Inyang M, Gao B, Pullammanappallil P, Ding W, Zimmerman AR (2010) Biochar from anaerobically digested sugarcane bagasse. Bioresour Technol 101(22):8868–8872
Inyang M, Gao B, Zimmerman A, Zhou Y, Cao X (2015) Sorption and cosorption of lead and sulfapyridine on carbon nanotube-modified biochars. Environ Sci Pollut Res 22:1868–1876
Inyanga MI, Gao B, Yao Y, Xue YW, Zimmerman A, Mosa A, Pullammanappallil P, Ok YS, Cao XD (2016) A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit Rev Env Sci Technol 46(4):406–433
Jacobs JA, Testa SM (2005) Overview of chromium(VI) in the environment: background and history. Chromium (VI) Handbook. CRC Press, Boca Raton, pp 1–22
Jarup L, Berglund M, Elinder CG (1998) Health effects of cadmium exposure—a review of the literature and a risk estimate published erratum appears in Scand. J Work Environ Health 24(3):240
Joseph SD, Downie A, Crosky A, Lehmann J, Munroe P (2007) Biochar for carbon sequestration, reduction of greenhouse gas emissions and enhancement of soil fertility; a review of the materials science. Rend Circ Mat Palermo Suppl 48:101–106
Jin JW, Li YA, Zhang JY, Wu SC, Cao YC, Liang P, Zhang J, Wong MH, Wang MY, Shan SD, Christie P (2016) Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J Hazard Mater 320:417–426
Kabata-Pendia A 3rd (ed) (2001) Trace elements in soils and plants. CRC Press, Boca Raton
Kalderis D, Kotti MS, Méndez A, Gascó G (2014) Characterization of hydrochars produced by hydrothermal carbonization of rice husk. Solid Earth 5:477–483
Kastner JR, Mani S, Juneja A (2015) Catalytic decomposition of tar using iron supported biochar. Fuel Process Technol 130:31–37
Keiluweit M, Nico PS, Johnson MG, Kleber M (2010) Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ Sci Technol 44:1247–1253
Kimetu JM, Lehmann J, Solomon ON, Mugendi DN, Kinyangi JM, Riha S, Verchot L, Recha JW, Pell AN (2008) Reversibility of soil productivity cecline with organic matter of differing quality along a degradation gradient. Ecosystems 11:726–739
Landolph J (1994) Molecular mechanisms of transformation of CH3/10T1/2C1 8 mouse embryo cells and diploid human fibroblasts by carcinogenic metal compounds. Environ Health Perspect 102:119–125
Langard S, Vigander T (1983) Occurrence of lung cancer in workers producing chromium pigments. J Ind Med 40(1):71–74
Laureano-Perez L, Teymouri F, Alizadeh H, Dale BE (2005) Understanding factors that limit enzymatic hydrolysis of biomass: characterization of pretreated corn stover. Appl Biochem Biotechnol 121–124:1081–1099
Lee HV, Hamid SBA, Zain SK (2014) Conversion of lignocellulosic biomass to nanocellulose: structure and chemical process. Sci World J 2014:1–20
Lee JW, Kidder M, Evans BR, Paik S, Buchanan AC, Garten CT, Brown RC (2010) Characterization of of biochars produced from cornstovers for soil amendment. Environ Sci Technol 44:7970–7974
Lehmann J, Gaunt J, Rondon M (2006) Bio-char sequestration in terrestrial ecosystems a review. Mitig Adapt Strat Glob Change 11:395–419
Lehmann J, Joseph S (2009) Biochar for environmental management: science and technology. Earthscan, UK
Leonardo WE, Franciele W, Antônio SM, Marcio V, Luís FM (2020) Facile method to prepare biochar–NiO nanocomposites as a promisor material for electrochemical energy storage devices. Chem Pap 74:1471–1476
Li R, Wang JJ, Gaston LA, Zhou BY, Li ML, Xiao R, Wang Q, Zhang ZQ, Huang HT, Zhang XF (2018a) An overview of carbothermal synthesis of metalebiochar composites for the removal of oxyanion contaminants from aqueous solution. Carbon 129:674–687
Li R, Wang JJ, Zhou B, Zhang Z, Liu S, Lei S, Xiao R (2017) Simultaneous capture removal of phosphate, ammonium and organic substances by MgO impregnated biochar and its potential use in swine wastewater treatment. J Clean Prod 147:96–107
Li XM, Shen QR, Zhang DQ, Mei XL, Ran W, Xu YC, Yu GH (2013) Functional groups determine biochar properties (pH and EC) as studied by two dimensional 13C NMR correlation spectroscopy. PLoS ONE 8:65949
Lima IM, Marshall WE (2005) Adsorption of selected environmentally important metals by poultry manure-based granular activated carbons. J Chem Technol Biotechnol 80:1054–1061
Liu SB, Tan XF, Liu YG, Gu YL, Zeng GM, Hu XJ, Wang H, Zhou L, Jiang LH, Zhao BB (2016) Production of biochars from Ca impregnated ramie biomass (Boehmeria nivea (L.)) and their phosphate removal potential. Rsc Adv 6(7):5871–5880
Li C, Zhang L, Gao Y, Li A (2018b) Facile synthesis of nano ZnO/ZnS modified biochar by directly pyrolyzing of zinc contaminated corn stover for Pb(II), Cu(II) and Cr(VI) removals. Waste Manag 79:625–637
Liu Z, Zhang FS (2009) Removal of lead from water using biochars prepared from hydrothermal liquefaction of biomass. J Hazard Mater 167:933–939
Liu WJ, Hong J, Han QY (2015) Development of biochar-based functional materials: toward a sustainable platform carbon material. Chem Rev 115:12251–12285
Lu L, Yu W, Wang Y, Zhang K, Zhu X, Zhang Y, Wu Y, Ullah H, Xiao X, Chen B (2020) Application of biochar-based materials in environmental remediation: from multi-level structures to specific devices. Biochar 2:1–31
Ma Y, Liu WJ, Zhang N, Li YS, Jiang H, Sheng GP (2014) Polyethylenimine modified biochar adsorbent for hexavalent chromium removal from the aqueous solution. Bioresour Technol 169:403–408
Maheswari CU, Reddy KO, Muzenda E, Guduri BR, Rajulu AR (2012) Extraction and characterization of cellulose microfibrils from agricultural residue—Cocos nucifera L. Biomass Bioenergy 46:555–563
Meyer S, Glaser B, Quicker P (2011) Technical, economical, and climate-related aspects of biochar production technologies: a literature review. Environ Sci Technol 45:9473–9483
Mohan D, Kumar H, Sarswat A, Alexandre-Franco M, Pittman CU (2014) Cadmium and lead remediation using magnetic oak wood and oak bark fast pyrolysis bio-chars. Chem Eng J 236:513–528
Mohan D, Pittman CU (2006) Activated carbons and low cost adsorbents for remediation of tri-and hexavalent chromium from water. Hazard Mater 137(2):762–811
Molino A, Chianese S, Musmarra D (2016) Biomass gasification technology: the state of the art overview. J Energy Chem 25(1):10–25
Mubarak N, Kundu A, Sahu J, Abdullah E, Jayakumar N (2014) Synthesis of palm oil empty fruit bunch magnetic pyrolytic char impregnating with FeCl3 by microwave heating technique. Biomass Bioenerg 61:265–275
Mumme J, Eckervogt L, Pielert J, Diakite M, Rupp F, Kern J (2011) Hydrothermal carbonization of anaerobically digested maize silage. Bioresour Technol 102:9255–9260
Nabajit DC, Rahul S, Chutia T, Bhaskar RK (2014) Pyrolysis of jute dust: effect of reaction parameters and analysis of products. J Mater Cycles Waste 16:449–459
Naeem A, Saddique MT, Mustafa S, Kim Y, Dilara B (2009) Cation exchange removal of Pb from aqueous solution by sorption onto NiO. J Hazard Mater 168:364–368
Nguyen BT, Lehmann J, Hockaday WC, Joseph S, Masiello CA (2010) Temperature sensitivity of black carbon decomposition and oxidation. Environ Sci Technol 44:3324–3331
Niazi NK, Bibi I, Shahid M, Ok YS, Burton ED, Wang HL, Shaheen SM, Rinklebe J, Lüttge A (2018) Arsenic removal by perilla leaf biochar in aqueous solutions and groundwater: an integrated spectroscopic and microscopic examination. Environ Pollut 232:31–41
Niazi NK, Bibi I, Shahid M, Ok YS, Shaheen SM, Rinklebe J, Wang HL, Murtaza B, Islam E, Bawaz F, Lüttge A (2018) Arsenic removal by Japanese oak wood biochar in aqueous solutions and well water: investigating arsenic fate using integrated spectroscopic and microscopic techniques. Sci Total Environ 621:1642–1651
Oladipo AA, Ifebajo AO (2018) Highly efficient magnetic chicken bone biochar for removal of tetracycline and fluorescent dye from wastewater: two-stage adsorber analysis. J Environ Manag 209:9–16
Pandey A, Bhaskar T, Stöcker M, Sukumaran R (2015) Recent advances in thermochemical conversion of biomass. Elsevier, Amsterdam
Park J, Choppala G, Bolan N, Chung J, Chuasavathi T (2011) Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 348:439–451
Patlolla A, Armstrong N, Tchounwou PB (2008) Cytogenetic evaluation of potassium dichromate toxicity in bone marrow cells of Sprague–Dawley rats. Metal Ions Biol Med 10:353–358
Patlolla A, Barnes C, Field J, Hackett D, Tchounwou PB (2009) Potassium dichromate-induced cytotoxicity, genotoxicity and oxidative stress in human liver carcinoma (HepG2) cells. Int J Environ Res Public Health 6:643–653
Puja K, Uzma D, Rout PK, Vinit Y, Shilpi J (2013) Plant refuses driven biochar: alication as metal adsorbent from acidic solutions. Arab J Chem 10:3054–3063
Rajapaksha AU, Alam MS, Chen N, Alessi DS, Igalavithana AD, Tsang DC, Ok YS (2018) Removal of hexavalent chromium in aqueous solutions using biochar: chemical and spectroscopic investigations. Sci Total Environ 625:1567–1573
Reddy DHK, Lee SM (2014) Magnetic biochar composite: facile synthesis, characterization, and application for heavy metal removal. Colloid Surface A 454:96–103
Rillig MC, Wagner M, Salem M, Antunes PM, George C, Ramke HG, Titirici MM, Antonietti M (2010) Material derived from hydrothermal carbonization: effects on plant growth and arbuscular mycorrhiza. Appl Soil Ecol 45:238–242
Roy P, Dias G (2017) Prospects for pyrolysis technologies in the bioenergy sector: a review. Renew Sustain Energy Rev 77:59–69
Rutherford DW, Wershaw RL, Rostad CE, Kelly CN (2012) Effect of formation conditions on biochars: compositional and structural properties of cellulose, lignin, and pine biochars. Biomass Bioenerg 46:693–701
Sandip M, Kaustav A, Kumar S, Krishna S, Rachana DC, Gulshan M, Gopinath H (2017) Optimizing ranitidine hydrochloride uptake of Parthenium hysterophorus derived biochar through response surface methodology and artificial neural network. Process Saf Environ Prot 107:388–401
Shinogi Y (2004) Nutrient leaching from carbon products of sludge. In: ASAE/CSAE Annual International Meeting, Ottawa, Ontario, Canada
Sakhiya AK, Anand A, Kaushal P (2020) Production, activation, and applications of biochar in recent times. Biochar 2:253–285
Shurong W, Gongxin D, Haiping Y, Zhongyang L (2017) Lignocellulosic biomass pyrolysis mechanism: a state-of-the-art review. Prog Energy Combust Sci 62:33–86
Singhal RL, Merali Z, Hrdina PD (1976) Aspects of the biochemical toxicology of cadmium. Fed Proc 35(1):75–80
Song Z, Lian F, Yu Z, Zhu L, Xing B, Qiu W (2014) Synthesis and characterization of a novel MnOx-loaded biochar and its adsorption properties for Cu2+ in aqueous solution. Chem Eng J 242:36–42
Stanislav VV, David B, Lars K, Andersen C, Vassileva G (2010) An overview of the chemical composition of biomass. Fuel 89(5):913–933
Stefanidis SD, Kalogiannis KG, Iliopoulou EF, Michailof CM, Pilavachi PA, Laas AA (2014) A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J Anal Appl Pyrolysis 105:143–150
Subedi R, Taupe N, Pelissetti S, Petruzzelli L, Bertora C, Leahy J, Grignani C (2016) Greenhouse gas emissions and soil properties following amendment with manure-derived biochars: influence of pyrolysis temperature and feedstock type. J Environ Manag 166:73–83
Suksabye P, Thiravetyan P (2012) Cr(VI) adsorption from electroplating plating wastewater by chemically modified coir pith. J Environ Manag 102(14):1–8
Tan X, Liu Y, Zeng G, Wang X, Hu X, Gu Y, Yang Z (2015) Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 125:70–85
Tang J, Lv H, Gong Y, Huang Y (2015) Preparation and characterization of a novel graphene/biochar composite for aqueous phenanthrene and mercury removal. Bioresour Technol 196:355–363
Titirici MM, White RJ, Falco C, Sevilla M (2012) Black perspectives for a green future: hydrothermal carbons for environment protection and energy storage. Energy Environ Sci 5:6796–6822
Tsai WT, Chang CV, Lee S (1997) Preparation and characterization of activated carbons from corn cob. Carbon 35:1198–2000
Uchimiya M, Lima IM, Klasson KT, Chang S, Wartelle LH, Rodgers JE (2010) Sorption of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter-derived biochars in water and soil. J Agri Food Chem 58:5538–5544
Verkleji JAS (1993) The effects of heavy metals stress on higher plants and their use as biomonitors. In: Markert B (ed) Plant as bioindicators: indicators of heavy metals in the terrestrial environment. VCH, New York, pp 415–424
Volotskova O, Levchenko I, Shashurin A, Raitses Y, Ostrikov K, Keidar M (2010) Single-step synthesis and magnetic separation of graphene and carbon nanotubes in arc discharge plasmas. Nanoscale 2:2281–2285
Wang H, Gao B, Wang S, Fang J, Xue Y, Yang K (2015) Removal of Pb(II), Cu(II), and Cd(II) from aqueous solutions by biochar derived from KMnO4 treated hickory wood. Bioresour Technol 197:356–362
Wang M, Sheng G, Qiu Y (2015) A novel manganese-oxide/biochar composite for efficient removal of lead (II) from aqueous solutions. Int J Environ Sci Technol 12:1719–1726
Wang J, Wang S (2019) Preparation, modification and environmental application of biochar: a review. J Cleaner Production 227:1002–1022
Wang L, Wang J, He C, Lyu W, Zhang W, Yan W, Yang L (2019) Development of rare earth element doped magnetic biochars with enhanced phosphate adsorption performance. Colloids Surf A 561:236–243
Wang S, Gao B, Zimmerman AR, Li Y, Ma L, Harris WG, Migliaccio KW (2015) Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour Technol 175:391–395
Jun W, Hong LJ, Han QY (2015) Development of biochar-based functional materials: toward a sustainable platform carbon material. Chem Rev 115:12251–12285
Wilson K, Yang H, Seo CW, Marshall WE (2006) Select metal adsorption by activated carbon made from peanut shells. Bioresour Technol 97:2266–2270
Xie T, Reddy K, Wang CW, Yargicoglu E, Spokas K (2015) Characteristics and applications of biochar for environmental remediation: a review. Crit Rev Environ Sci Technol 45:939–969
Xu Y, Chen B (2013) Investigation of thermodynamic parameters in the pyrolysis conversion f biomass and manure to biochars using thermogravimetrisc analysis. Bioresour Technol 146:485–493
Xuan L, Yang Z, Zifu L, Rui F, Yaozhong Z (2014) Characterization of corncob derived biochar and pyrolysis kinetics in comparison with corn stalk and sawdust. Bioresour Technol 170:76–82
Yan L, Kong L, Qu Z, Li L, Shen G (2014) Magnetic biochar decorated with ZnS nanocrytals for Pb (II) removal. ACS Sustain Chem Eng 3:125–132
Yang Q, Wang X, Luo W, Sun J, Xu Q, Chen F, Zhao J, Wang S, Yao F, Wang D, Li X, Zeng G (2018) Effectiveness and mechanisms of phosphate adsorption on iron modified biochars derived from waste activated sludge. Bioresour Technol 247:537–544
Yao Y, Gao B, Chen J, Yang L (2013) Engineered biochar reclaiming phosphate from aqueous solutions: mechanisms and potential application as a slow-release fertilizer. Environ Sci Technol 47:8700–8708
Yao Y, Gao B, Inyang M, Zimmerman AR, Cao X, Pullammanappallil P, Yang L (2011) Removal of phosphate from aqueous solution by biochar derived from anaerobically digested sugar beet tailings. J Hazard Mater 190(1):501–507
Yang Z, Shen J, Jayaprakash N, Archer LA (2012) Synthesis of organic−inorganic hybrids by miniemulsion polymerization and their application for electrochemical energy storage. Energy Environ Sci 5:7025
Yu KL, Lau BF, Show PL (2017) Recent developments on algal biochar production and characterization. Bioresour Technol 246:2–11
Yuan JH, Xu RK, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour Technol 102:3488–3497
Yuan Z, Cheng X, Zhong L, Wu R, Zheng Y (2019) Preparation, characterization and performance of an electrospun carbon nanofiber mat applied in hexavalent chromium removal from aqueous solution. J Environ Sci 77:75–84
Zhang H, Voroney RP, Price GW (2015) Effects of temperature and processing conditions on biochar chemical properties and their influence on soil C and N transformations. Soil Biol Biochem 83:19–28
Zhang M, Gao B (2013) Removal of arsenic, methylene blue, and phosphate by biochar/AlOOH nanocomposite. Chem Eng J 226:286–292
Zhang M, Gao B, Varnoosfaderani S, Hebard A, Yao Y, Inyang M (2013) Preparation and characterization of a novel magnetic biochar for arsenic removal. Bioresour Technol 130:457–462
Zhang M, Gao B, Yao Y, Xue Y, Inyang M (2012a) Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions. Chem Eng J 210:26–32
Zhang S, Ji Y, Dang J, Zhao J, Chen S (2019) Magnetic apple pomace biochar: simple preparation, characterization, and application for enriching Ag(I) in effluents. Sci Total Environ 668:115–123
Zhang M, Gao B, Yao Y, Xue Y, Inyang M (2012b) Synthesis, characterization, and environmental implications of graphene-coated biochar. Sci Total Environ 435:567–572
Zhao X, Liu W, Cai Z, Han B, Qian T, Zhao D (2016) An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation. Water Res 100:245–266
Acknowledgements
The authors of this paper are thankful to the Central University of Jammu for providing all the required facilities for carrying this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
All authors and co-authors have followed the ethical guidelines prescribed by the Journal.
Conflict of interest
Authors and co-authors do not have any conflicts of interest.
Informed consent
All authors and co-authors have given their consent for publication of the manuscript in this journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pathania, D., Srivastava, A.K. Advances in nanoparticles tailored lignocellulosic biochars for removal of heavy metals with special reference to cadmium (II) and chromium (VI). Environmental Sustainability 4, 201–214 (2021). https://doi.org/10.1007/s42398-020-00142-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42398-020-00142-w