Abstract
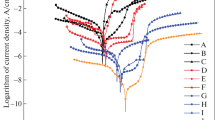
Aluminum-iron activated with ore tailing alloy was developed as an anode for the protection of steel in a seawater environment using an impressed current cathodic protection mechanism. The materials used in this research were aluminum, iron ore tailings and low-carbon steel. The anode was produced via the sand-casting method by the addition of varying weight fractions of iron ore tailings (5–25%). The resulting anodes were characterized; the microstructure of the anodes was observed by scanning electron microscopy/energy dispersive spectroscopy, and X-ray diffraction was used to identify the phases present. The corrosion behavior of the anodes was determined in seawater with a salinity of 2.1% using a potentiostat. The micrograph results showed that some anodes had large grains, small grains and a mixture of unevenly distributed grains throughout the material. The energy dispersive spectroscopy spectrogram showed aluminum, oxygen and zinc to be the most prominent elements in the anodes. In addition, inclusions and several intermetallics were observed. Sample D (85 Al% + 15 IOT%) has a corrosion potential of -405.197 mV (silver/silver chloride) compared to steel, which has a corrosion potential of -637.653 mV (silver/silver chloride). Sample D has more positive potentials than steel, which means that sample D is the most suitable anode for protecting steel via impressed current system cathodic protection.














Similar content being viewed by others
Data Availability
All the data employed in support of the outcome of the study are included in this article.
References
Ikubanni PP, Oki M, Adeleke AA, Onaolapo AS, Paramasivam P (2023a) Corrosion behaviour of Al/SiC/PKSA hybrid composites in 1.0 M Sulphuric acid environment. Eng Rep e12733. https://doi.org/10.1002/eng2.12733
Ikubanni PP, Adeleke AA, Odusote JK, Adegoke H, Oki M, Okolie JA (2023b) Corrosion control of AISI 1007 steel using hybrid inhibitors of plant extracts. Chem Afr 6(6):3161–3171
Odusote JK, Adeleke AA, Ikubanni PP, Asafa TB, Kolawole SK, Opatola EA, Orhadahwe TA (2024) Corrosion inhibition potential of silver-gold nanoparticles on mild steel in 1.0 M hydrochloric acid. Chem Afr 7(1):243–256
Parthiban GT, Parthiban T, Ravi R, Saraswathy V, Palaniswamy N, Sivan V (2008) Cathodic protection of steel in concrete using magnesium alloy anode. Corros Sci 50(12):3329–3335
Szabo S, Bakos I (2006) Cathodic protection with sacrificial anodes. Corros Rev 24:231–280
Craig BD, Lane RA, Rose DH (2006) Corrosion prevention and control: a program management guide for selecting materials. Advanced Materials, Manufacturing, and Testing Information Analysis Center (AMMTIAC)
Grundmeier G, Schmidt W, Stratmann M (2000) Corrosion protection by organic coatings: electrochemical mechanism and novel methods of investigation. Elect Acta 45:2515–2533
Crevello G, Noyce P (2014) Preserving a memorial: The use of impressed current cathodic protection on a historic steel frame rotunda. In: Corrosion 2014, San Antonio, Texas, USA
Peabody AW (2001) In: Bianchetti RL, Editor (eds) Peabody’s control of pipeline corrosion, 2nd edn. NACE International, Houston, Texas
Song Y, Du C, Li X (2006) Electrochemical corrosion behavior of carbon steel with bulk coating holidays. J Univ Sci Technol Beijing Mineral Metallur Mat 13(1):37–43
Ashworth V (2010) 4.18. Principles of cathodic protection. Shreir’s Corros. Elsevier, New York, NY, USA, pp 2747–2762
Bhuiyan S (2015) Effectiveness of impressed current cathodic protection systems in concrete following current interruption (Doctoral dissertation), RMIT University
Zhao XJ, Zhu G, Fan YJ, Li HY, Wang ZL (2015) Triboelectric charging at the nanostructured solid/liquid interface for area-scalable wave energy conversion and its use in corrosion protection. ACS Nano 9(7):7671–7677
Shibli SMA, Jabeera B, Manu R (2007) Development of high-performance aluminium alloy sacrificial anodes reinforced with metal oxides. Mat Lett 61:3000–3004
Deng ZY, Liu YF, Tanaka Y, Ye J, Sakka Y (2005) Modification of Al particle surfaces by γ-Al2O3 and its effect on the corrosion behaviour of Al. J Am Ceramic Soc 88(4):977–979
Popoola API, Olorunniwo OE, Ige OO (2014) Corrosion resistance through the application of anti-corrosion coatings. In: developments in corrosion protection. InTech Open 241–270. https://doi.org/10.5772/57420
Galedari SA, Mahdavi A, Azarmi F, Huang Y, McDonald A (2019) A comprehensive review of corrosion resistance of thermally-sprayed and thermally-diffused protective coatings on steel structures. J Therm Spray Tech 28:645–677
Munoz AG, Saidman SB, Bessone JB (2002) Corrosion of an Al–Zn–In alloy in chloride media. Corros Sci 44(10):2171–2182
Hamad MF (2015) Cathodic protection of carbon steel using aluminium as sacrificial anode in sea water. Al-Nahrain J Eng Sci 18(1):91–100
Ameh ES, Ikpeseni SC (2017) Pipelines cathodic protection design methodologies for impressed current and sacrificial anode systems. Nigerian J Tech 36(4):1072–1077
Loto CA, Popoola API (2011) Effect of anode and size variations on the cathodic protection of mild steel in sea water and sulphuric acid. Int J Phys Sci 6(12):2861–2868
Toriola-Coker LO, Ajimo AO, Obisanya AA (2021) Effect of saline water on the compressive strength of concrete. J Sci Eng Res 5(1):15–23
Castro-Sastre MÁ, García-Cabezón C, Fernández-Abia AI, Martín-Pedrosa F, Barreiro J (2021) Comparative study on microstructure and corrosion resistance of Al-Si alloy cast from sand mold and binder jetting mold. Metals 11(9):1421
Yao J, Yang Y, Li Y, Jiang J, Xiao S, Yang J (2021) Interconnected α-Fe2O3 nanoparticles prepared from leaching liquor of tin ore tailings as anode materials for lithium-ion batteries. J Alloys Compd 855:157288. https://doi.org/10.1016/j.jallcom.2020.157288
Tamasgavabari R, Ebrahimi AR, Abbasi SM, Yazdipour AR (2020) Effect of harmonic vibration during gas metal arc welding of AA-5083 aluminium alloy on the formation and distribution of intermetallic compounds. J Manufact Process 49:413–422
Rajasekhar K, Harendranath CS, Raman R, Kulkarni SD (1997) Microstructural evolution during solidification of austenitic stainless-steel Weld metals: a color metallographic and electron microprobe analysis study. Mat Characteriz 38(2):53–65
Awan GH, Ul Hasan F (2008) The morphology of coating/substrate interface in hot-dip-aluminized steels. Mat Sci Eng A 472:157–165
Hashimoto K, Asami K, Kawashima A, Habazaki H, Akiyama E (2007) The role of corrosion resistant alloying elements in passivity. Corros Sci 49(1):42–52
Wang F, Xu J, Xu Y, Jiang L, Ma G (2020) A comparative investigation on cathodic protections of three sacrificial anodes on chloride-contaminated reinforced concrete. Construct Building Mat 246:118476
Talebian M, Raeissi K, Atapour M, Fernández-Pérez BM, Betancor-Abreu A, Llorente I, Fajardo S, Salarvand Z, Meghdadi S, Amirnasr M, Souto RM (2019) Pitting corrosion inhibition of 304 stainless steel in NaCl solution by three newly synthesized carboxylic Schiff bases. Corros Sci 160:108130
Funding
The authors received no funding from any source.
Author information
Authors and Affiliations
Contributions
This work was carried out in collaboration between all authors. Authors: Daniel Toyin Oloruntoba, Olanrewaju Seun Adesina, Babatunde Iwarere designed the study, performed the experiment, interpreted results and wrote the first draft of the manuscript. Authors: Olanrewaju Seun Adesina, Olufemi Oluseun Sanyaolu, Peter Pelumi Ikubanni, and Adeolu Adesoji Adediran managed the analyses of the study, managed literature searches and graphical editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests with any previous work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oloruntoba, D.T., Adesina, O.S., Sanyaolu, O.O. et al. Development of Aluminium Base Anode for Cathodic Protection of Steel. Chemistry Africa (2024). https://doi.org/10.1007/s42250-024-00962-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42250-024-00962-x