Abstract
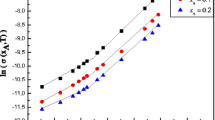
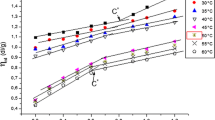
The present work aims to study the effect of precipitant (ethanol) addition on the polyelectroyte conformation dissolved in water. Viscosity measurements are used to study the behavior of polysufonate sodium (PSS) of molar mass, Mw (g/mol) = 1,000,000, in water/ethanol mixture at 298.15 K in dilute and semi-dilute regimes. In fact, the specific viscosity of the prospected solutions and alcohol mixture composition, xA, and for all the polyelectrolyte concentration range are determined. The overlapping concentration, C*(xA) (g/dl), was deduced for the different mixture composition. This quantity decreases by increasing the quantity of ethanol in the mixture. In the dilute concentration regime, the intrinsic viscosity of PSS and the interamolecular interaction parameter were determined according to the Fuoss equation. The obtained results show a decrease of the intrinsic viscosity when ethanol is added. In the semi-dilute regime, the relative viscosity was determined and the Flory exponent was deduced for PSS in the studied water/ethanol mixture showing that the polyelectrolyte takes different conformation.



Similar content being viewed by others
References
Chakraborty G, Bhattarai A, De R (2022) Polyelectrolyte–dye interactions: an overview. Polymers 14(3):598–625. https://doi.org/10.3390/polym14030598
Teraoka I (2002) Polymer solutions: an introduction to physical properties. John Wiley & Sons, Inc., New Jersey
Kamli M, Guettari M, Tajouri T (2019) Structure of polyvinylpyrrolidone aqueous solution in semi-dilute regime: roles of polymer–surfactant complexation. J Mol Struct 1196:176–185. https://doi.org/10.1016/j.molstruc.2019.06.069
El Aferni A, Guettari M, Kamli M, Tajouri T, Ponton A (2020) A structural study of a polymer–surfactant system in dilute and entangled regime: effect of high concentrations of surfactant and polymer molecular weight. J Mol Struct 1199:127052. https://doi.org/10.1016/j.molstruc.2019.127052
Aferni AEL, Guettari M, Tajouri T (2016) Effect of polymer conformation on polymer–surfactant interaction in salt-free water. Colloid Polym Sci 294:1097–1103. https://doi.org/10.1007/s00396-016-3869-8
Belaidi A, Guettari M, Tajouri T (2019) Gamma-ray irradiation-induced variations in structural and electrical properties of PVP neutral polymer in water. J Radioanal Nucl Chem 322:869–877. https://doi.org/10.1007/s10967-019-06803-3
Wilts EM, Herzberger J, Long TE (2018) Addressing water scarcity: cationic polyelectrolytes in water treatment and purification. Polym Int 67:799–814. https://doi.org/10.1002/pi.5569
Picos-Corrales LA, Licea-Claverie A, Sarmiento-Sánchez JI, Ruelas-Leyva JP, Osuna-Martínez U, García-Carrasco M (2023) Methods of nanoencapsulation of phytochemicals using organic platforms. In: Basilio Heredia J, Gutiérrez-Grijalva EP, Licea-Claverie A, Gutierrez-Uribe JA, Patra JK (eds) Nanotechnology in biomedicine phytochemical nanodelivery systems as potential biopharmaceuticals. Elsevier, Amsterdam, pp 123–184
Hössel P, Dieing R, Nörenberg R, Pfau A, Sander R (2000) Conditioning polymers in today’s shampoo formulations—efficacy, mechanism and test methods. Int J Cosmet Sci 22(1):1–10. https://doi.org/10.1046/j.1467-2494.2000.00003.x
Zoghlami O, Guettari M, Tajouri T (2017) Study of poly (sodium-4-styrenesulfonate) behavior in water/non-solvent mixtures by conductivity and refractive index measurements. Colloid Polym Sci 295:1729–1739. https://doi.org/10.1007/s00396-017-4104-y
Gheorghe AM, Mihaela M, Dan Cristian V (2019) The use of chitosan, alginate, and pectin in the biomedical and food sector—biocompatibility, bioadhesiveness, and biodegradability. Polymers 11:1837. https://doi.org/10.3390/polym11111837
Yaowen L, Saeed A, Dur ES, Yue W, Rui L, Jianwu D, Suqing L, Wen Q (2021) A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci Technol 112:532–546. https://doi.org/10.1016/j.tifs.2021.04.016
Yan M, Qu L, Fan J et al (2014) Electrostatic complexation of polyelectrolyte and magnetic nanoparticles: from wild clustering to controllable magnetic wires. Nanoscale Res Lett 9:198. https://doi.org/10.1186/1556-276X-9-198
Vis M, Peters VFD, Erne BH, Tromp RH (2015) Ion entropy in phase-separated aqueous mixtures of polyelectrolyte and neutral polymer. Macromolecules 48(8):2819–2828. https://doi.org/10.1021/acs.macromol.5b00324
Zheng Y, Lin C, Zhang JS et al (2020) Ion-mediated interactions between like-charged polyelectrolytes with bending flexibility. Sci Rep 10:21586. https://doi.org/10.1038/s41598-020-78684-6
Spruijt E, Martien A, Stuart C, van der Gucht J (2010) Dynamic Force spectroscopy of oppositely charged polyelectrolyte brushes. Macromolecules. 43(3):1543–1550. https://doi.org/10.1021/ma902403a
Takeaki A (2010) Conformational changes of polyelectrolyte chains in solvent mixtures. Soft Matter. https://doi.org/10.1039/c6sm00352d
Caplan SPC, Silva TBG, Franscisco ADS, Lachter ER, Nascimento RSV (2019) Sulfonated polystyrene nanoparticles as oleic acid diethanolamide surfactant nanocarriers for enhanced oil recovery processes. Polymers 11(9):1513. https://doi.org/10.3390/polym11091513
Manning GS (1972) Polyelectrolytes. Annu Rev Phys Chem 23(1):117–140. https://doi.org/10.1146/annurev.pc.23.100172.001001
Colby RH, Boris DC, Krause WE, Tan JS (1997) Polyelectrolyte conductivity. J Polym Sci B Polym Phys 35(17):2951–2960. https://doi.org/10.1002/(SICI)1099-0488(199712)35:17%3c2951::AID-POLB18%3e3.0.CO;2-6
Guettari M, Zoghlami O, Tajouri T (2022) Deviation of the manning theory predictions from the electrical conductivity measurements of a polyelectrolyte in its solvent/non-solvent mixture. Chem Afr 3:771–780. https://doi.org/10.1007/s42250-021-00309-w
Lin KF, Cheng HL (2000) A simple method to estimate chain conformations of polyélectrolytes in the semidilute regime. Macromolecules 33(13):4961–4965. https://doi.org/10.1021/ma991766q
Guettari M, Gomati R, Gharbi A (2012) Determination of the flory exponent by study of steady shear viscosity. J Macromol Sci B Phys 51:153–163. https://doi.org/10.1080/00222348.2011.564087
Acknowledgements
The authors gratefully acknowledge fnancial support from the Tunisian Ministry of Education, Research, and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zoghlami, O., Guettari, M. & Tajouri, T. Effect of Precipitant (Ethanol) Addition on the Conformation of Polysulfonate Sodium in Water. Chemistry Africa 7, 2189–2194 (2024). https://doi.org/10.1007/s42250-023-00861-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00861-7