Abstract
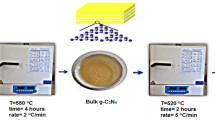
Novel zeolite-based porous carbon (ZC) composites derived during direct pyrolysis of chitosan and polyacrylic acid as carbon sources and of zeolite as a template followed by acid soaking of HCl and HF were studied. Characterization tools including SEM, XRD, FTIR and N2 adsorption–desorption measurements were employed to assess the effect of acid soaking on the surface and adsorption properties of products (ZC-HCl and ZC-HF). Adsorption of methylene blue (MB) cationic dye was examined to evaluate the adsorption capacities of ZC-HCl and ZC-HF composites. SEM, XRD and FTIR analyses proved that soaking with HCl acid (ZC-HCl) could be removed large amounts of zeolite and formed functional groups with positively charges on the carbon surface. Whereas soaking by HF acid (ZC-HF) influenced slightly on the surface and morphology of zeolite-carbon composite with maintaining negatively charges confirming the attachment of zeolite on the carbon surface. Total surface areas as high as 170 and 241 m2/g were obtained for ZC-HCl and ZC-HF, respectively. Langmuir isotherm could be fitted the adsorption of dye. It was found that equilibrium adsorption capacities of MB at 25 °C and pH 6 were 45.5 and 83.6 mg/g onto ZC-HCl and ZC-HF, respectively. Kinetic data were well-described by pseudo-second order model and consequently the chemisorption powered the adsorption process over the sample's surface. Therefore, the obtained ZC-HF sample is considered to be a potential adsorbent for removing cationic dyes.









Similar content being viewed by others
Data availability
All data available on request.
References
Chung DDL (2019) Carbon materials: science and applications. World Scientific Publishing (ISBN 9789813221901)
Fathy NA, El-Shafey SE, El-Shafey OI (2017) Synthesis of a novel MnO2@carbon nanotubes-graphene hybrid catalyst (MnO2@CNT-G) for catalytic oxidation of basic red 18 dye (BR18). J Water Proc Eng 17:95–101
El-Khouly SM, Fathy NA, Farag HK, Aboelenin RMM (2020) In2O3 catalyst supported on carbonaceous nanohybrid for enhancing the removal of methyl orange dye from aqueous solutions. Des Water Treat 174:344–353
Annamalai KP, Fathy NA, Tao Y (2017) Synthesis and capacitance performance of phosphorous-enriched carbon xerogel. J Sol-Gel Sci Technol 48:515–521
Iqbal S, Khatoon H, Pandit AH, Ahmad S (2019) Recent development of carbon based materials for energy storage devices. Mater Sci Energy Technol 2:417–428
Xia Y, Walker GS, Grant DM, Mokaya R (2009) Hydrogen storage in high surface area carbons: experimental demonstration of the effects of nitrogen doping. J Am Chem Soc 131:16493–16499
Alam N, Mokaya R (2011) Characterization and hydrogen storage of Pt-doped carbons templated by Ptex changed zeolite Y. J Micropor Mesopor Mater 142:716–724
Susanti I, Widiastuti N (2019) Activation of zeolite-Y templated carbon with KOH to enhance the CO2 adsorption capacity. Malay J Fund Appl Sci 15:249–253
Masika E, Mokaya R (2013) Preparation of ultra high surface area porous carbons templated using zeolite 13X for enhanced hydrogen storage. Prog Natl Sci Mater Internat 23:308–316
Nishihara H, Kyotani T (2018) Zeolite-templated carbons—three-dimensional microporous graphene frameworks. Chem Commun 54:5648–5673
Pavlenko V, Khosravi SH, Zołtowska S, Haruna AB, Zahid M, Mansurov Z, Supiyeva Z, Galal A, Ozoemena KI, Abbas Q, Jesionowski T (2022) A comprehensive review of template-assisted porous carbons: modern preparation methods and advanced applications. Mater Sci Eng R 149:100682
Duraisamy V, Selvakumar K, Krishnan R, Murugesan S, Kumar S (2019) Investigation on template etching process of SBA-15 derived ordered mesoporous carbon on electrocatalytic oxygen reduction reaction. ChemistrySelect 4:2463–2474
Katsuki H, Furuta S, Watari T, Komarneni S (2005) ZSM-5 zeolite/porous carbon composite: conventional- and microwave-hydrothermal synthesis from carbonized rice husk. Micropor Mesopor Mater 86:145–151
Wongcharee S, Aravinthan V, Erdei L (2019) Mesoporous activated carbon-zeolite composite prepared from waste macadamia nut shell and synthetic faujasite. Chin J Chem Eng 27:226–236
Cheng WP, Gao W, Cui X, Ma JH, Li RF (2016) Phenol adsorption equilibrium and kinetics on zeolite X/activated carbon composite. J Taiwan Inst Chem Eng 62:192–198
Xin G, Wang M, Chen L, Zhang Y, Wang M, Jiang W, Chen Y (2019) Synthesis and properties of zeolite/N-doped porous carbon for the efficient removal of chemical oxygen demand and ammonia-nitrogen from aqueous solution. RSC Adv 9:6452–6459
Chai Z, Lv P, Bai Y, Wang J, Song X, Su W, Yu G (2022) Low-cost Y-type zeolite/carbon porous composite from coal gasification fine slag and its application in the phenol removal from wastewater: fabrication, characterization, equilibrium, and kinetic studies. RSC Adv 12:6715–6724
Maheshwari K, Agrawal M, Gupta AB (2021) Dye pollution in water and wastewater. In: Muthu SS, Khadir A (eds) Novel materials for dye-containing wastewater treatment. Sustainable textiles: production, processing, manufacturing and chemistry. Springer, Singapore
Fathy NA, El-Khouly SM, Sayed Ahmed SA, El-Nabarawy TA, Tao Y (2021) Superior adsorption of cationic dye on novel bentonite/carbon composites. Asia-Pac J Chem Eng 16:e2586
Ghaedi M, Golestani NA, Khodadoust S, Rajabi M, Azizian S (2014) Application of activated carbon as adsorbents for efficient removal of methylene blue: kinetics and equilibrium study. J Ind Eng Chem 20:2317–2324
Fathy NA, El-Khouly SM, Aboelenin RMM (2019) Carbon xerogel/carbon nanotubes nanohybrid doped with Ti for removal of methylene blue dye, Egypt. J Chem 62:2277–2288
Fathy NA, El-Shafey OI, Khalil LB (2013) Effectiveness of alkali–acid treatment in enhancement the adsorption capacity for Rice straw: the removal of methylene blue dye. ISRN Phys Chem 2013:1–15
Jamil TS, Abdel Ghafar HH, Ibrahim HS, Abd El-Maksoud IH (2011) Removal of methylene blue by two zeolites prepared from naturally occurring Egyptian kaolin as cost effective technique. Solid State Sci 13:1844–1851
Dolas H (2023) Activated carbon synthesis and methylene blue adsorption from pepper stem using microwave assisted impregnation method: isotherm and kinetics. J King Saud Univ Sci 35:102559
Wang J, Ma J, Sun Y (2022) Adsorption of methylene blue by coal-based activated carbon in High-Salt Wastewater. Water 14(21):3576
Yan C, Wang C, Yao J, Zhang L, Liu X (2009) Adsorption of methylene blue on mesoporous carbons prepared using acid- and alkaline-treated zeolite X as the template. Coll Surf A Physicochem Eng Aspects 333:115–119
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–470
Temkin MI, Pyzhev V (1940) Kinetic of ammonia synthesis on promoted iron catalyst. Acta Phys Chem URSS 12:327–356
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. 591. Kungliga Svenska Vetenskapsakademiens, Handlingar 24:1–39
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div Am Soc Civ Eng 89:31–60
Xia K, Li Ch, Zhao S, Li Y, Duan L, Liu X (2022) Effect of HF and NaOH etching on the composition and structure of SiOC ceramics. Ceram Int 48:1789–1795
Ndlovu N, Missengue R, Petrik LF, Ojumu T (2017) Synthesis and characterization of Faujasite zeolite and geopolymer from South African coal fly ash. J Environ Eng 143:04017042
Mozgawa W, Król M, Dyczek J, Deja J (2014) Investigation of the coal fly ashes using IR spectroscopy. Spectrochim Acta Part A Mol Biomol Spectrosc 132:889–894. https://doi.org/10.1016/j.saa.2014.05.052
Hajiraissi R, Parvinzadeh M (2011) Preparation of polybutylene terephthalate/silica nanocomposites by melt compounding: evaluation of surface properties. Appl Surf Sci 257:8443–8450
Neolaka YAB, Lawa Y, Naat J, Riwu AAP, Mango AW, Darmokoesoemo H, Widyaningrum BA, Iqbal M, Kusuma HS (2022) Efficiency of activated natural zeolite-based magnetic composite (ANZ-Fe3O4) as a novel adsorbent for removal of Cr(VI) from wastewater. J Mater Res Technol 18:2896–2909
Mohamed GM, Ahmed SAS, Fathy NA (2023) Activated carbon doped with silica and nitrogen as novel adsorbent for enhancing adsorption capacity of Cr(VI). Chem Afr. https://doi.org/10.1007/s42250-023-00709-0
Almasian A, Gashti MP, Olya ME, Fard GC (2016) Poly (acrylic acid)-zeolite nanocomposites for dye removal from single and binary systems. Desalin Water Treatment. https://doi.org/10.1080/19443994.2015.1112841
Acknowledgements
This work was carried out using the technical support of National Research Centre, Egypt.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All experimental and analyses were made equally. Dr. Sh. El-Shafey wrote the experimental part, prepared all calculations and figures. Prof. N. Fathy Wrote the main manuscript. Both authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interest.
Ethical Approval
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fathy, N.A., El-Shafey, S. Effect of Acid Soaking on Properties of Zeolite-Based Porous Carbon Composite Prepared for Adsorption of Cationic Dye. Chemistry Africa 7, 1535–1545 (2024). https://doi.org/10.1007/s42250-023-00826-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00826-w