Abstract
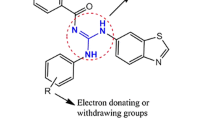
Twelve new analogues of gabapentin (GBP) derived hydrazide-hydrazone scaffolds were produced by using various electronically and structurally divergent aromatic aldehydes. All the hydrazones were obtained in moderate to good yields (66–82%) by heating the GBP hydrazide with respective aldehydes to a temperature of 45–65 °C for 4–7 h. Further, the compounds were explored for their antimicrobial activity on three different Gram positive (Micrococcus luteus, Streptococcus mutans, Enterococcus faecalis) and Gram negative bacterial strains (Salmonella enterica, Alcaligenes faecalis, Pseudomonas aeruginosa). The antibacterial results obtained were found to be good against Gram positive bacteria when compared to Gram negative bacteria. Moreover, the compounds were also screened for antioxidant activity at four different concentrations using the DPPH method and the results showed that some of the compounds were moderately active.


Similar content being viewed by others
Availability of data and material (data transparency)
Yes.
Code availability
(Software application or custom code): CS Chem draw Ultra.
References
Goa KL, Sorkin EM (1993) Gabapentin: a review of its pharmacological properties and clinical potential in epilepsy. Drugs 46:409–427. https://doi.org/10.2165/00003495-199346030-00007
Chen J, Li L, Chen S-R, Hong C, Xie J-D, Sirrieh RE, MacLean DM, Zhang Y, Zhou M-H, Jayaraman V, Pan H-L (2018) The α2d-1-NMDA receptor complex is critically involved in neuropathic pain development and gabapentin therapeutic actions. Cell Rep 22:2307–2321. https://doi.org/10.1016/j.celrep.2018.02.021 (and references cited theirin)
Smith RV, Havens JR, Walsh SL (2016) Gabapentin misuse, abuse and diversion: a systematic review. Addiction 111:1160–1174. https://doi.org/10.1111/add.13324
Wu W-P, Hao J-X, Ongini E, Impagnatiello F, Presotto C, Wiesenfeld-Hallin Z, Xu X-J (2004) A nitric oxide (NO)-releasing derivative of gabapentin, NCX 8001, alleviates neuropathic pain-like behavior after spinal cord and peripheral nerve injury. J Pharmacol 141(1):65–74. https://doi.org/10.1038/sj.bjp.0705596
Ahmad N, Subhan F, Islam NU, Shahid M, Rahman FU, Robert D, Sewell E (2017) Gabapentin and its salicyladehyde derivative alleviate allodynia and hypoalgesia in a cisplatin-induced neuropathic pain model. Eur J Pharmacol 814:302–312. https://doi.org/10.1016/j.ejphar.2017.08.040
Schwarz JB, Gibbons SE, Graham SR, Colbry NL, Guzzo PR, Le V-D, Vartanian MG, Kinsora JJ, Lotarski SM, Li Z, Dickerson MR, Su T-Z, Weber ML, El-Kattan A, Thorpe AJ, Donevan SD, Taylor CP, Wustrow DJ (2005) Novel cyclopropyl beta-amino acid analogues of pregabalin and gabapentin that target the alpha2-delta protein. J Med Chem 48(8):3026–3035. https://doi.org/10.1021/jm0491086
Chen C, Stearns B, Hu T, Naomi Anker N, Santini A, Arruda JM, Campbell BT, Datta P, Aiyar J, Munoz B (2006) Expedited SAR study of high-affinity ligands to the α2δ subunit of voltage-gated calcium channels: Generation of a focused library using a solution-phase Sn2Ar coupling methodology. Bioorg Med Chem Lett 16(3):746–749. https://doi.org/10.1016/j.bmcl.2005.08.117
Hussain EA, Kanwal N, Khan IU, Mutahir S, Khan MA, Ahmed M, Mahmood MA, Sahin O, Akkurt M, Yar M (2018) Green and facile reaction of gabapentin with sulfonyl chlorides to synthesize lactams and sulfonamides derivatives in aqueous medium. Lett Org Chem 15:163–170. https://doi.org/10.2174/1570178614666170907143340
Akat H, Balcan M (2007) Synthesis and characterization of polymethacrylamide having gabapentin moieties. Iranian Poly J 16(11):769–774
Katuri JVP, Ekkundi VS, Nagarajan K (2016) A simple and expedient procedure for the preparation of gabapentin lactam (2-aza-spiro [4,5] decan-3-one). Org Proc Res Dev 20:1828–1832. https://doi.org/10.1021/acs.oprd.6b00246
Khajehali N, Darehkordi A (2020) Gabapentin-based synthesis of novel oxo- and spiro-dihydroquinazoline derivatives. J Iran Chem Soc 17:433–440. https://doi.org/10.1007/s13738-019-01779-z
Popiołek L (2017) Hydrazide-hydrazones as potential antimicrobial agents: overview of the literature since 2010. Med Chem Res 26:287–301. https://doi.org/10.1007/s00044-016-1756-y
Bedia K-K, Elçin O, Seda U, Fatma K, Nathaly S, Sevim R, Dimoglo A (2006) Synthesis and characterization of novel hydrazide-hydrazones and the study of their structure-antituberculosis activity. Eur J Med Chem 41(11):1253–1261. https://doi.org/10.1016/j.ejmech.2006.06.009
Kumar P, Narasimhan B (2013) Hydrazides/hydrazones as antimicrobial and anticancer agents in the new millennium. Mini Rev Med Chem 13(7):971–987. https://doi.org/10.2174/1389557511313070003
Shabeeb I, Al-Essa L, Shtaiwi M, Al-Shalabi E, Younes E, Okasha R, Sini MA (2019) New hydrazide-hydrazone derivatives of quinoline 3-carboxylic acid hydrazide: synthesis, theoretical modeling and antibacterial evaluation. Lett Org Chem 16(5):430–436. https://doi.org/10.2174/1570178616666181227122326
Yatcherla SR, Islam A, Nageswar D, Bollikolla HB (2015) Synthesis, characterization and antibacterial activity of some new 3-(3- (trifluoromethyl) phenyl)-3-(2-hydroxy-5-methylphenyl)propanehydrazones. Indian J Chem Sec B 54(9):1162–1167
Yatcherla SR, Islam A, Nageswar D, Bollikolla HB (2015) Synthesis, characterization and antibacterial activity of some new hydrazide-hydrazone derivatives linked with bezafibrate scaffold. Int J Curr Res Chem Pharma Sci 2(2):25–30
Ramya Krishna P, Sailaja G, Umamaheswara Rao V, Hari Babu B (2020) Bezafibrate scaffold derived hydrazide-hydrazones: synthesis and antioxidant activities. Egypt J Chem 63(7):3–5. https://doi.org/10.21608/EJCHEM.2020.20809.2251
Kaur M, Yogita T, Rana RC (2005) Antimicrobial properties of Achyranthes aspera. Anc Sci Life 24(4):168–173
Chew AL, Jessica JJA, Sasidharan S (2012) Antioxidant and antibacterial activity of different parts of Leucas aspera. Asian Pac J Trop Biomed 2(3):176–180. https://doi.org/10.1016/S2221-1691(12)60037-9
Acknowledgements
The first author specially thank Acharya Nagarjuna University for providing University Research Fellowship. The 3&6 authors thank CSIR for chemicals support (FNo. 02(0198)/EMR-II dt 14.11.2014).
Author information
Authors and Affiliations
Contributions
RKP, BRM and BMK are actively involved in work; URV in pharmacological evaluation; HB and RV in manuscript writing and total proof work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pallapati, R.K., Mutchu, B.R., Khandapu, B.M.K. et al. Synthesis of Novel Gabapentin Scaffold Derived Hydrazide-hydrazones for Potential Antimicrobial agents and Antioxidants. Chemistry Africa 3, 881–888 (2020). https://doi.org/10.1007/s42250-020-00184-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-020-00184-x