Abstract
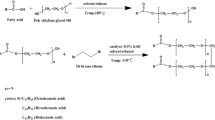
Series of novel non-ionic Gemini surfactant was prepared in two steps. Initially diethanolamine was converted into amide by using fatty acids viz. lauric acid, palmitic acid, myristic acid and stearic acid and corresponding prepared amide reacted with 1,6-dibromohexane to form the non-ionic Gemini surfactants. The formation of the targeted molecule was monitored and confirmed using FTIR, 1H-NMR and 13C-NMR spectroscopy. The effect of alkyl chain length of synthesized surfactants on the basic characteristics such as surface tension, foaming, emulsifying capability, lime soap dispersing power and wetting were studied. These new surfactants were investigated for their anticorrosion and biological properties. They have capacity to reduce surface tension of water from 71.8 to 25.44 mN m−1. All the Gemini surfactants possessed good antimicrobial activity against gram positive Bacteria Bacillis subtilis and Staphylococcus aureus. The N,N-bis(hydroxyethyl)octadecanamide based Gemini surfactant have 94.11% corrosion inhibition efficiency against carbon steel. They have low biodegradability.
Graphic Abstract







Similar content being viewed by others
References
El-Shamy OAA, Khid TT, Doheim MM (2011) Effect of ethoxylate chain length on the pour point depressant of middle distillate fuel oil. J Dispers Sci Technol 32:654–658
Schmitt MT (2001) Analysis of surfactants. Surfactant science series, 3rd edn. CRC Press, Boca Raton, p 96
Becher P (1990) In: Basel JMR (ed) A review of cationic surfactants: organic chemistry, surfactant science series, vol 34, 1st edn. Marcel Dekker Inc, New York
Gunstone FD (1999) Fatty acid and lipid chemistry, vol 1. Aspen Publisher Inc., Gaithersburg
Channouf RB, Souissi N, Zanna S, Ardelean H, Bellakhal N, Marcus P (2018) Surface characterization of the corrosion product layer formed on synthetic bronze in aqueous chloride solution and the effect of the adding of juniperus communis extract by X-ray photoelectron spectroscopy analysis. Chem Afr 1:167–174
Jan J, Margareta S, Britt-Marie L, Andreas A, Tore C, Bengt R (2003) A synthetic surfactant based on a poly-Leu SP-C analog and phospholipids: effects on tidal volumes and lung gas volumes in ventilated immature newborn rabbits. J Apply Physiol 95:2055–2063
Han L, Chen H, Luo P (2004) Viscosity behavior of cationic gemini surfactants with long alkyl chains. Surf Sci 564:141–148
Devinsky F, Lacko I, Bittererova F, Tomeckova LJ (1986) Relationship between structure, surface activity and micelle formation of some new bisquaternary isosteres of 1,5-pentanediammonium dibromides. J Colloid Interface Sci 114:314–322
Okahara M, Masuyama A, Sumida Y, Zhu YP (1988) Surface active properties of new types of amphipathic compounds with two hydrophilic ionic groups and two lipophilic alkyl chains. J Jpn Oil Chem Soc 37:746–748
Mangat CK, Kaur S (2014) Synthesis, characterization and surface properties of cationic gemini surfactant. J Dispers Sci Technol 35:1528–1556
Zang Y, Yongshen X, Shouji Q, Yang L (2013) Synthesis and properties of mono or double long-chain alkanolamine surfactants. J Surfact Deterg 16:841–848
Patil VK, Gawali IT, Usmani GA (2016) Synthesis and properties of novel cationic triazolium gemini surfactants. J Dispers Sci Technol 37:1630–1637
Gawali IT, Mali Punam, Usmani GA (2016) 4,5-Dihydroimidazoline based non-ionic gemini surfactants: synthesis, characterization, physicochemical properties and anticorrosion behaviour in 1 N H2SO4 aqueous medium. Res J Recent Sci 5:39–49
Gawali IT, Umani GA (2018) Monoethanolamide based non-ionic gemini surfactants from renewable sources: synthesis, characterization and anticorrosion study. Int Educ J Sci Eng 1:12–20
Gawali IT, Umani GA (2019) Synthesis, surface active properties and applications of cationic gemini surfactants from triethylenetetramine. J Dispers Sci Technol. https://doi.org/10.1080/01932691.2019.1584112
Schwartz, Perry (1949) Surface active agents, their chemistry and technology. Interscience Publishers Inc, New York, pp 175–183
Dai Y et al (2017) Synthetic method of coconut diethanol amide. China patent CN107513025A
Williamson AW (1852) On etherification. J Chem Soc 4:229–239
Baylis RL, Bevan TH, Malkin T (1958) The synthesis of cephalin (phosphatidylethanolamine) and batyl, chimyl, glycol, and alkyl analogues. J Chem Soc. https://doi.org/10.1039/JR9580002962
Smith RG, Vanterpool A, Culak HJ (1969) Dimethyl sulfoxide as a solvent in Williamson ether synthesis. Can J Chem 47:2015–2019
Ahmad I, Patial P, Kaur C, Kaur S (2014) Cationic imidazolium monomeric surfactants: their synthesis and surface active properties. J Surfact Deterg 17:269–277
Hughes FA, Lew BW (1970) Physical and functional properties of some higher alkyl polyglucosides. J Am Oil Chem Soc 47:162–167
Gawali IT, Usmani GA (2014) Study of physico-chemical properties of glycerol ester based non-ionic gemini surfactant. Int J Sci Res 3:580–584
McCutecheon JW (1950) Synthetic detergents. McNair-Dorland’s, New York
Zhu YP, Masuyama A, Okahara M (1990) Preparation and surface active properties amphipathic compounds with two sulfate groups and two lipophilic alkyl chains. J Am Oil Chem Soc 67:459–463
Wakasman SA, Lechevalver HA (1962) The actinomycetes antibiotic of actinomycetes, vol 430. Williams & WIkins Co., Baltimore
El-Sukkary MMA, Syed NA, Helmy SM, Ismail A, El-Azab WIM (2009) Aqueous solution properties, biodegradability and antimicrobial activity of some alkylpolyglycosides surfactants. Tenside Surf Det 46:311–316
Petre ET, Richard RE, David A, Gayle KM, Felix HO (1974) Degradable surfactants derived from corn starch. JAOCS 51:486–494
Fouda AS, Elewady YA, Abd El-Aziz HK (2012) Corrosion inhibition of carbon steel by cationic surfactants in 0.5 m HCl solution. J Chem Sci Technol 1:45–53
Lah J, Pohar C, Vesnaver G (2000) Calorimetric study of the micellization of alkylpyridinium and alkyltrimethylammonium bromides in water. J Phys Chem B 104:2522–2526
Ismayilov IT, Abd El-Lateef HM, Abbasov VM, Aliyeva LI, Qasimov EE, Efremenko EN, Ismayilov TA, Mamedxanova SA (2013) Inhibition effects of some novel surfactants based on corn oil and diethanolamine on mild steel corrosion in chloride solutions saturated with CO2. Int J Thin Film Sci Technol 2:91–105
Li ZX, Dong CC, Thomas RK (1999) Neutron reflectivity studies of the surface excess of gemini surfactants at the air–water interface. Langmuir 15:4392–4396
Varma SK, Gosh KK (2011) Micellar and surface properties of some monomeric surfactants and a gemini cationic surfactant. J Surfact Deterg 14:347–352
Yeshimua T, Bong M, Matsuoka K, Honda C, Endo K (2009) Surface properties and aggregate morphology of partially fluorinated carboxylate-type anionic gemini surfactants. J Colloid Interface Sci 339:230–235
Xin L, Zhiyong H, Hailin Z, Duanlin C (2010) Synthesis and properties of novel alkyl sulfonates gemini surfactants. J Surfact Deterg 13:353–359
Shahin M, Hady SA, Hammad MN (2011) Mortadadevelopment of stable O/W emulsions of three different oils. Int J Pharm Stud Res 2:45–51
Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma MJ (2005) Nano-emulsions. Curr Opin Colloid Interface Sci 10:102–110
Toliwal SD, Patel K (2007) Preparation and surfactant properties of diethanolamides of rice bran, soyabean and rapeseed protein. J Sci Ind Res 66:385–387
Ware AM, Waghmare JT, Momin SA (2007) Alkylpolyglycoside: carbohydrate based surfactant. J Dispers Sci Technol 28:437–444
Adewale A, Rotimi AO, Rao AVSK, Prasad RBN (2012) Synthesis of alkanolamide: nonionic surfactant from the oil of Gliricida sepium. J Surfact Deterg 15:89–96
Qun X, Liyan W, Fenglan X (2011) Synthesis and properties of dissymmetric gemini surfactants. J Surfact Deterg 14:85–90
Karsa DR, Porter MR (1995) Biodegradability of surfactants. Springer, Netherlands
Tundo PP, Anastas D, Black BJ, Collins T, Memoli S, Miyamoto J, Polyakoff M, Tumas W (2000) Synthetic pathways and processes in green chemistry. Introductory overview. Pure Apply Chem 72:1207–1228
Sheng Z, Lijing G, Linlin Z (2011) Synthesis and properties of a new piperazine-based bicaudate gemini surfactant. J Dispers Sci Technol 33:960–964
Adewale A, Rotomi AO, Adebobola OA (2013) Antimicrobial activity of non-ionic and anionic surfactants from Citrullus lantus seed oil. Jundishapur J Microbiol 6:205–209
Bradshaw LJ (1992) Laboratory microbiology. Saunders College, New York
Raja PB, Sethuraman MG (2008) Inhibition effect of pepper extract on the sulphuric acid corrosion of mild steel. Mater Lett 62:2977–2979
Tang LB, Mu GN, Liu GH (2003) The effect of neutral red on the corrosion inhibition of cold rolled steel in 1.0 m hydrochloric acid. Corros Sci 45:2251–2262
Farag AA, Noor El-Din MR (2012) The adsorption and corrosion inhibition of some nonionic surfactants on API X65 steel surface in hydrochloric acid. Corros Sci 64:174–183
Free ML (2002) Understanding the effect of surfactant aggregation on corrosion inhibition of mild steel in acidic medium. Corros Sci 44:2865–2870
Heakal FE, Deyab FMA, Osman MM, Nessimb MI, Elkholyb AE (2017) Synthesis and assessment of new cationic gemini surfactants as inhibitors for carbon steel corrosion in oilfield water. RSC Adv 7(75):47335–47352
Ozturk S, Mudaber S, Yıldırım A (2018) Synthesis of 2,3-dihydroxypropyl-sulfanyl derivative nonionic surfactants and their inhibition activities against carbon steel corrosion in acidic media. JOTCSA 5(2):333–346
Acknowledgements
This work was supported by University Grants Commission (UGC), India [Project F. no. 41-373/2012 (SR)].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gawali, I.T., Usmani, G.A. Novel Non-ionic Gemini Surfactants from Fatty Acid and Diethanolamine: Synthesis, Surface-Active Properties and Anticorrosion Study. Chemistry Africa 3, 75–88 (2020). https://doi.org/10.1007/s42250-019-00107-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-019-00107-5