Abstract
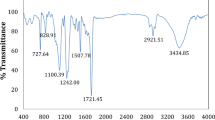
In this study, TiO(OH)2/MnO2/PEG12000 composite was elaborated on pure copper in a two-step deposition process in the presence of 1% w/v of polyethylene glycol 12000 (PEG12000). The influence of PEG12000 on the composite morphology, composition and surface roughness was studied by SEM–EDS and AFM techniques. The results show that a porous composite of a multilayer structure with PEG12000 dispersed spherical particles was synthesized. Though the roughness homogeneity of TiO(OH)2/MnO2/PEG12000 was slightly improved, carbon content ranges from 5 to 12 At% reflecting an adsorption process of PEG12000 during the composite deposition. Results were confirmed by XPS technique, which provides further evidence about the existence of PEG12000. High-resolution C1s and O1s signals affirm the presence of the characteristic C–O bond, resulting from the coupling of PEG12000 on the composite surface. The electrochemical corrosion was evaluated in chloride medium by OCP measurements and linear polarisation curves. It was found that the corrosion resistance of TiO(OH)2/MnO2 composite was improved markedly in the presence of PEG12000. The stability of TiO(OH)2/MnO2/PEG12000 composite was also studied by following the evolution of impedance parameters during immersion time in 0.05 M NaCl medium completed by a characterization of the composite morphology and composition at the end of immersion by SEM–EDS and XPS analysis. The composite indicates good stability due to the formation of PEG-CuCl complexes, capable of limiting the infiltration of Cl− ions in both the composite and the substrate surfaces.


















Similar content being viewed by others
References
Besghaier R, Dhouibi L, Jeannin M, Safi MJ (2019) Chem Afr 2:1–13
Channouf RB, Souissi N, Zanna S, Ardelean H, Bellakhal N, Marcus P (2018) Chem Afr 1:167–174
Hefnawy A, Elkhoshkhany N, Essam A (2018) J Alloy Compd 735:600–606
Karimi MA, Aghaei VH, Nezhadali A, Ajami N (2019) J Mater Sci Mater Electron 30:6300–6310
Kumari S, Panigrahi A, Singh SK, Pradhan SK (2018) J Coat Technol Res 15:583–592
Tatsuma T, Saitoh S, Ohko Y (2001) Fujishima. Chem Mater 13:2838–2842
Subasri R, Shinohara T (2003) Electrochem Commun 5:897–902
Boulares A, Dhouibi L, Berçot P, Rezrazi EM (2018) Chem Afr 1:127–144
Boudellioua H, Hamlaoui Y, Tifouti L, Pedraza F (2019) Appl Surf Sci 473:449–460
Fouda AS, El-Dossoki FI, Shady IA (2018) Green Chem Lett Rev 11:67–77
El-Lateef HMA (2016) Res Chem Intermed 42:3219–3240
Kabir H, Nandyala SH, Rahman MM, Kabir MA, Pikramenou Z, Laver M, Stamboulis A (2019) Ceram Int 45:424–431
Chakrabarty B, Ghoshal AK, Purkait MK (2008) J Membr Sci 309:209–221
Ballesteros J, Díaz-Arista P, Meas Y, Ortega R, Trejo G (2007) Electrochim Acta 52:3686–3696
Wang X, Cai S, Liu T, Ren M, Huang K, Zhang R, Zhao H (2014) Ceram Int 40:3389–3398
Afroukhteh S, Dehghanian C, Emamy M (2012) Progr Nat Sci Mater Int 22:480–487
Nina Balès (2014) LES PEGS (Monograph, University of Quebec in Chicoutimi). http://www.uqac.ca. Accessed 1 Jan 2015
Yougui L (2018) Practical electron microscopy and database. www.Globalsino.Com/EM/. Accessed 13 Dec 2018
Shard AG, Spencer SJ (2019) Surf Interface Anal 51:618–626
Jin Y, Sun M, Mu D, Ren X, Wang Q, Wen L (2012) Electrochim Acta 78:459–465
Jin Y, Kondo K, Suzuki Y, Matsumoto T, Barkey DP (2005) Electrochem Solid State Lett 8:C6–C8
XPS, AES, UPS and ESCA. laSurface.com. http://www.lasurface.com/database/elementxps.php. Accessed 18 June 2019
Landoulsi J, Genet MJ, Fleith S, Touré Y, Liascukiene I, Méthivier C, Rouxhet PG (2016) Appl Surf Sci 383:71–83
Blacha-Grzechnik A, Piwowar K, Zdyb T, Krzywiecki M (2018) Appl Surf Sci 457:221–228
Dai L, Xu D (2019) Tetrahedron Lett 60:1005–1010
Sun Q, Luo Y, Xiang P, Yang X, Shen M (2017) Forensic Sci Int 277:1–9
Simpson DE, Johnson CA, Roy DJ (2019) Electrochem Soc 166:D3142–D3154
Hebert KR (2005) J Electrochem Soc 152:C283–C287
Gründer Y, Stettner J, Magnussen OM (2019) J Electrochem Soc 166:D3049–D3057
Lee H, Tsai ST, Wu PH, Dow WP, Chen CM (2019) Mater Charact 147:57–63
Zhu QS, Zhang X, Liu CZ, Liu HY (2019) J Electrochem Soc 166:D3006–D3012
Mroczka R, Łopucki R, Żukociński G (2019) Appl Surf Sci 463:412–426
Kear G, Barker BD, Stokes KR, Walsh FC (2007) Electrochim Acta 52:1889–1898
Bard AJ, Faulkner LR (2001) Electrochemical methods: fundamentals and applications, 2nd edn. Wiley, New York
Chang T, Leygraf C, Wallinder IO, Jin Y (2019) J Electrochem Soc 166:D10–D20
Song SJ, Choi SR, Kim JG (2019) J Electroanal Chem 832:75–86
Biesinger MC (2017) Interface Anal 49:1325–1334
Orazem ME, Frateur I, Tribollet B, Vivier V, Marcelin S, Pébère N, Bunge AL, White EA, Riemer DP, Musiani M (2013) J Electrochem Soc 160:C215–C225
Song HK, Hwang HY, Lee KH, Dao LH (2000) Electrochim Acta 45:2241–2257
Rezaei Niya SM, Hoorfar M (2016) Electrochim Acta 188:98–102
Orazem ME, Pébère N, Tribollet B (2006) J Electrochem Soc 153:B129
Orazem ME (2013) ECS Trans 50:247–260
Augustin C (2008) Prévision Des Cinétiques de Propagation de Défauts de Corrosion Affectant Les Structures En Alliage d’aluminium 2024 (PhD Thesis, Toulouse University). http://ethesis.inp-toulouse.fr. Accessed 21 Nov 2012
Ciccola A, Guiso M, Domenici F, Sciubba F, Bianco A (2017) Polym Degrad Stab 140:74–83
Wang L, Liu G, Xue D (2011) Electrochim Acta 56:6277–6283
Nicholson AP, Hemenway DR, Sampath WS, Barth KL (2017) J Vac Sci Technol A Vac Surf Films 35:041511
Zhong Y, Li Y, Li S, Feng S, Zhang Y (2014) Rsc Advances 4:40638–40642
Acknowledgements
The authors would like to thank the financial support provided by “Action Intégrée Franco-Tunisienne du Ministère des Affaires Etrangères et Européennes français et du Ministère de l’Enseignement Supérieur et de la Recherche Scientifique tunisien”. The authors acknowledge Dr. Olivier HEINTZ for XPS measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Boulares, A., Dhouibi, L., Berçot, P. et al. Effect of Polyethylene Glycol 12000 on Morphology and Corrosion Behavior of TiO(OH)2/MnO2/PEG12000 Composite Electrodeposited on Pure Copper. Chemistry Africa 2, 645–661 (2019). https://doi.org/10.1007/s42250-019-00085-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-019-00085-8