Abstract
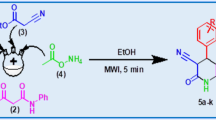
A series of novel indole derivatives bearing pyrimidine and cyclic imide scaffolds such as phthalic and maleic anhydrides has been designed and synthesized using both conventional and microwave irradiation (MW) methods under solvent free conditions. The title compounds have been developed by the reaction of 2-aminoo-4-hydroxy-6-(5,1-substituted-indol-3-yl) pyrimidine-5-carbonitrile with phthalic and maleic anhydrides individually using MW method. In addition, these target compounds were also synthesised under conventional heating method. A considerable increase in the reaction rate has been observed with better yields (90–92%) within 2–6 min using microwave irradiation in comparison to conventional thermal treatment.
Graphic Abstract





Similar content being viewed by others
References
Buu Bui HT, Kim Ha QT, Won KO, Duy DV, Tram Chau YN, Kim Tu CT, Pham EC, Tran PT, Tran LT, Mai HV (2016) Microwave assisted synthesis and cytotoxic activity evaluations of new benzimidazole derivatives. Tetrahedron Lett 57:887–891
Sharma P, Reddy TS, Kumar NP, Senwar KR, Bhargava SK, Shankaraiah N (2017) Conventional and microwave-assisted synthesis of new 1Hbenzimidazole-thiazolidinedione derivatives: a potential anticancer scaffold. Eur J Med Chem 138:234–245
Desai NC, Satodiya HM, Rajpara KM, Joshi VV, Vaghani HV (2017) A microwave-assisted facile synthesis of novel coumarin derivatives containing cyanopyridine and furan as antimicrobial agents. J Saudi Chem Soc 21:S153–S162
Shaikh IN, Bagwan UF, Hunagund SM (2018) Cu-catalyzed rapid synthesis of novel fluorinated indole derivatives under microwave irradiation. Chem Africa 1:3–9
Raval JP, Desai KG, Desai KR (2006) Neat reaction technology for the synthesis of 4-oxo-thiazolidines derived from 2-SH-benzothiazole and antimicrobial screening of some synthesized 4-thiazolidinones. J Iran Chem Soc 3:233–241
Raval JP, Desai JT, Desai CK, Desai KR (2008) A comparative study of microwave assisted and conventional synthesis of 2,3-dihydro-2-aryl-4-[4-(2-oxo-2H-chromen-3-yl)-1,3-thiazol-2-ylamino]-1,5-benzothiazepines and its antimicrobial activity. Arkivoc xii:233–244
Raval JP, Desai KR (2009) A comparative study of microwave-assisted and conventional synthesis of novel 2-(4-diethylamino-2-hydroxyphenyl)-3-substituted-thiazolidin-4-one derivatives. Lietuvos Mokslu Akademija 20:101–108
Raval JP, Patel HV, Patel PS, Patel NH, Desai KR (2009) A rapid convenient microwave assisted and conventional synthesis of novel azetidin-2-one derivatives as potent antimicrobial agents. Asian J Res Chem 2:171–177
Al-Hazimi HM, El-Faham A, Ghazzali M, Al-Farhan K (2012) Microwave irradiation: a facile, scalable and convenient method for synthesis of N-phthaloylamino acids. Arab J Chem 5:285–289
Ghazzali M, El-Faha A, Abd-Megeed A, Al-Farhan K (2012) Microwave-assisted synthesis, structural elucidation and biological assessment of 2-(2-aceta-midophenyl)-2-oxo-N-phenyl acetamide and N-(2-(2-oxo-2(phenylamino) acetyl) phenyl)propionamide derivatives. J Mol Struct 1013:163–167
Charde M, Shukla A, Bukhariya V, Mehta J, Chakole R (2012) A review on: a significance of microwave assist technique in green chemistry. Int J Phytopharm 2:39–50
Ravichandran S, Karthikeyan E (2011) Microwave synthesis—a potential tool for green chemistry. Int J Chem Tech Res 3:466–470
Farghaly AAH (2010) Synthesis of some new indole derivatives containing pyrazoles with potential antitumor activity. Arkivoc 11:177–187
Singh OM, Thokchom SP (2017) Recent progress in biological activities of indole and indole alkaloids. Mini Rev Med Chem 18(1):9–25
Sharma V, Kumar P, Pathaka K (2010) Biological importance of the indole nucleus in recent years: a comprehensive review. J Heterocycl Chem 47:491–502
Zhu GD, Gandhi VB, Gong J, Luo Y, Liu X, Shi Y, Guan R, Magnone SR, Klinghofer V, Johnson EF, Bouska J, Shoemaker A, Oleksijew A, Jarvis K, Park C, Jong RD, Oltersdorf T, Li Q, Rosenberg SH, Giranda VL (2006) Discovery and SAR of oxindole–pyridine-based protein kinase B/Akt inhibitors for treating cancers. Bioorg Med Chem Lett 16:3424–3429
He L, Chang H, Chou TC, Savaraj N, Cheng CC (2003) Design of antineoplastic agents based on the ‘2-phenylnaphthalene-type’ structural pattern-synthesis and biological activity studies of 1H-indolo[3.2-c] quinoline derivatives. Eur J Med Chem 38:101–107
Xu L, Russu WA (2013) Molecular docking and synthesis of novel quinazoline analogues as inhibitors of transcription factors NF-κB activation and their anti-cancer activities. Bioorg Med Chem 23:540–546
Nagarapu L, Vanaparthi S, Bantu R, Kumar CG (2013) Synthesis of novel benzo [4,5] thiazolo [1,2-a] pyrimidine-3-carboxylate derivatives and biological evaluation as potential anticancer agents. Eur J Med Chem 69:817–822
Jain KS, Chitre TS, Miniyar PB, Kathiravan MK, Bendre VS, Veer VS, Shahane SR, Shishoo C (2006) Biological and medicinal significance of pyrimidines. J Curr Sci 90:793–803
Ballell L, Robert AF, Chung GAC, Young R (2007) New thiopyrazolo [3,4-d]pyrimidine derivatives as anti-mycobacterial agents. Bioorg Med Chem Lett 17:1736–1740
Gorlitzer K, Herbig S, Walter RD (1997) Indeno [1,2-d]pyrimidin-4-ylamine. Pharmazie 52:670–672
Malik V, Singh P, Kumar S (2006) Unique chlorine effect in regioselective one-pot synthesis of 1-alkyl-/allyl-3-(o-chlorobenzyl) uracils: anti-HIV activity of selected uracil derivatives. Tetrahedron 62:5944–5951
Ungureanu M, Moldoveanu CC, Poeata A, Drochioiu G, Petrovanu M, Mangalagiu I (2006) Nouveaux dérivés pyrimidiniques doués d’activité antibactérienne ou fongistatique in vitro. Ann Pharm Fr 64:286–288
Wagner E, Al-Kadasi K, Zimecki M, Sawka Dobrowolska W (2008) Synthesis and pharmacological screening of derivatives of isoxazolo[4,5-d]pyrimidine. Eur J Med Chem 43:2498–2504
Miyazaki Y, Matsunaga S, Tang J, Maeda Y, Nakano M, Philippe RJ, Shibahara M, Liu W, Sato H, Wang L, Notle RT (2005) Novel 4-amino-furo[2,3-d]pyrimidines as Tie-2 and VEGFR2 dual inhibitors. Bioorg Med Chem Lett 15(9):2203–2207
Nassar E (2010) Synthesis, (in vitro) antitumor and antimicrobial activity of some pyrazoline, pyridine and pyrimidine derivatives linked to indole moiety. J Am Sci 6(8):463–471
Zahran MAH, Ibrahim AM (2009) Synthesis and cellular cytotoxicities of new N-substituted indole-3-carbaldehyde and their indolylchalcones. J Chem Sci 121:455–462
Biradar JS, Somappa SB (2014) 2,5-Disubstituted novel indolyl pyrimidine analogues as potent antimicrobial agents. Der Pharm Lett 4:344–348
Saundane AR, Yarlakatti M, Walmik P, Katkar V (2012) Synthesis, antioxidant and antimicrobial evaluation of thiazolidinone, azetidinone encompassing indolylthienopyrimidines. J Chem Sci 124:469–481
Mohamed MS, Youns MM, Ahmed NM (2014) Novel indolylpyrimidine derivatives: synthesis, antimicrobial, and antioxidant evaluations. Med Chem Res 23:3374–3388
Prajapti SK, Nagarsenkar A, Guggilapu SD, Gupta KK, Allakonda L, Jeengar MK, Naidu VGM, Babu BN (2016) Synthesis and biological evaluation of oxindole linked indolyl-pyrimidine derivatives as potential cytotoxic agents. Bioorg Med Chem Lett 26:3024–3028
Teisseire H, Vernet G (2001) Effects of the fungicide folpet on the activities of antioxidative enzymes in duckweed. Pestic Biochem Physiol 69(2):112–117
Orzeszko A, Kaminska B, Starosciak BJ (2002) Synthesis and antimicrobial activity of new adamantine derivatives III. Farmaco 57:619–624
Chapman JM, Cocolas GH, Hall IH (1979) Hypolipidemic activity of phthalimide derivatives. 1. N-Substituted phthalimide derivatives. J Med Chem 22:1399–13402
Sena VL, Srivastava RM, Silva RO, Lima VL (2003) Synthesis and hypolipidemic activity of N-substituted phthalimides. Farmaco 58:1283–1288
Casaban-Ros E, Anton-Fos GM, Galvez J, Duart MJ, Garcia Domenech R (1999) Search for new antihistaminic compounds by molecular connectivity. Quant Struct Act Relatsh 18:35–43
Wiecek M, Kiec-Kononowicz K (2009) Synthesis and anticonvulsant evaluation of some N-substituted phthalimides. Acta Pol Pharm 66(4):249–255
Vamecq J, Lambert D, Poupaert JH, Masereel B, Stables JP (1998) Anticonvulsant activity and interactions with neuronal voltage-dependent sodium channel of analogues of ameltolide. J Med Chem 41:3307–3313
Shinji C, Nakamura T, Maeda S, Yoshida M, Hashimoto Y, Miyachi H (2005) Design and synthesis of phthalimide-type histone deacetylase inhibitors. Bioorg Med Chem Lett 15:4427–4431
Ungwitayatorn J, Wiwat C, Matayatsuk C, Pimthon J, Piyaviriyakul S (2008) Synthesis and HIV-1 reverse transcriptase inhibitory activity of non-nucleoside phthalimide derivatives. Chin J Chem 26(2):379–384
Hargreaves MK, Pritchard JG, Dave HR (1970) Cyclic carboxylic monoimides. Chem Rev 70:439–469
Shibata Y, Sasaki K, Hashimoto Y, Iwasaki S (1996) Phenylphthalimides with tumor necrosis factor alpha production-enhancing activity. Chem Pharm Bull 44:156–162
Axel GG, Jörg N, Photoinduced AD (2011) Electron-transfer chemistry of the bielectrophoric N-phthaloyl derivatives of the amino acids tyrosine, histidine and tryptophan. Beilstein J Org Chem 7:518–524
Hurd CD, Prapas AG (1959) Preparation of acyclic imides. J Org Chem 24:388–392
Walker MA (1995) A high yielding synthesis of N-alkyl maleimides using a novel modification of the mitsunobu reaction. J Org Chem 60:5352–5355
Aubert MT, Farnier M, Guilard R (1991) Reactivity of iminophosphoranes towards some symmetrical dicarbonyl dichlorides: syntheses and mechanisms. Tetrahedron 47:53
Sena VL, Srivastava RM, Silva RO, Lima VL (2003) Synthesis and hypolipidemic activity of N-substituted phthalimides. Farmaco 58:1283–1288
Vasilevskaya TN, Yakovleva OD, Kobrin VSA (1995) A convenient method of N-methylphthalimide synthesis. Synth Commun 25:2463–2465
Dabiria M, Salehib P, Baghbanzadeha M, Shakouria M, Otokesha S, Ekramia T, Doostia R (2007) Efficient and eco-friendly synthesis of dihydropyrimidinones, bis(indolyl)methanes and N-alkyl and N-arylimides in ionic liquids. J Iran Chem Soc 4:393–401
Zhou MY, Li YQ, Xu XM (2003) A new simple and efficient synthesis of N-aryl phthalimides in ionic liquid [bmim] [PF6]. Synth Commun 33:3777–3780
Liang J, Lv J, Fan JC, Shang ZC (2009) Polyethylene glycol as a nonionic liquid solvent for the synthesis of N-alkyl and N-arylimides. Synth Commun 39:2822–2828
Gupta R, Jain A, Madan Y, Menghani E (2014) A “One Pot”, environmentally friendly, multicomponent synthesis of 2-amino-5-cyano-4-[(2-aryl)-1H-indol-3-yl]-6-hydroxypyrimidines and their antimicrobial activity. J Heterocycl Chem 51:1395–1403
Acknowledgements
The authors are thankful to the authorities of Jawaharlal Nehru Technological University, Hyderabad, for providing laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bakkolla, M.G., Taduri, A.K. & Bhoomireddy, R.D. A Facile and Microwave Assisted Solvent Free Synthesis of Novel Indole Pyrimidine Imide Derivatives. Chemistry Africa 2, 587–595 (2019). https://doi.org/10.1007/s42250-019-00083-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-019-00083-w