Abstract
Fluorinated indoles have received considerable attention because incorporation of fluorine into target molecule can influence reactivity, selectivity and biological activity. Herein, a simple microwave-assisted synthesis of novel fluorinated indole derivatives have been developed by the reaction of 5-fluoroindoline-2,3-dione with various anilines. The reaction could be conducted using readily available substrates within short periods of 9–15 min under microwave irradiation with good to excellent yields of the product (64–92%). This approach exploits the synthetic potential of microwave irradiation and copper dipyridine dichloride (CuPy2Cl2) combination and offers many advantages such as full reaction control, excellent product yields, shorter reaction time, eco-friendly procedure and rapid feedback.
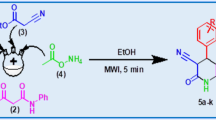
Graphical Abstract


Similar content being viewed by others
References
Buu Bui HT, Kim Ha QT, Won KO, Duy DV, Tram Chau YN, Kim Tu CT, Pham EC, Tran PT, Tran LT, Mai HV (2016) Microwave assisted synthesis and cytotoxic activity evaluations of new benzimidazole derivatives. Tetrahedron Lett 57:887–891
Sharma P, Reddy TS, Kumar NP, Senwar KR, Bhargava SK, Shankaraiah N (2017) Conventional and microwave-assisted synthesis of new 1Hbenzimidazole-thiazolidinedione derivatives: a potential anticancer scaffold. Eur J Med Chem 138:234–245
Desai NC, Satodiya HM, Rajpara KM, Joshi VV, Vaghani HV (2017) A microwave-assisted facile synthesis of novel coumarin derivatives containing cyanopyridine and furan as antimicrobial agents. J Saudi Chem Soc 21:S153–S162
Bálint E, Keglevich G (2016) The spread of the application of the microwave technique in organic synthesis. In: Keglevich G (ed) Milestones in microwave chemistry, Switzerland: Springer International Publishing. pp 1–10. (ISBN: 978-3-319-30630-8)
Kiss NZ, Bálint E, Keglevich G (2016) Microwave-assisted syntheses in organic chemistry. In: Keglevich G (ed) Milestones in microwave chemistry, Switzerland: Springer International Publishing. pp 11–46. (ISBN: 978-3-319-30630-8)
Polshettiwar V, Varma RS (2008) Microwave-Assisted Organic Synthesis and Transformations using Benign Reaction Media. Acc Chem Res 41:629–639
Caddick S, Fitzmaurice R (2009) Microwave enhanced synthesis. Tetrahedron 65:3325–3355
Lidstrom P, Tierney J, Wathey B, Westman J (2001) Microwave assisted organic synthesiste. Trahedron 57:9225–9283
Arya K, Dandia A (2007) Synthesis of biologically important novel fluorinated spiro heterocycles under microwaves catalyzed by montmorillonite KSF. J Fluorine Chem 128:224–231
Arya K, Rawat DS, Dandia A, Sasai H (2012) Bronsted acidic ionic liquids: green, efficient and reusable catalyst for synthesis of fluorinated spiro [indole-thiazinones/thiazolidinones] as antihistamic agents. J Fluorine Chem 137:117–122
Liu XH, Weng JQ, Wang BL, Li YH, Tan CX, Li ZM (2014) Microwave-assisted synthesis and biological activity study of novel fluorinated 1,2,4-triazole derivatives. Res Chem Intermed 40:2605–2612
Dandia A, SinghR Sachdeva H, Arya K (2001) Microwave assisted one pot synthesis of a series of trifluoromethyl substituted spiro [indole–triazoles]. J Fluorine Chem 111:61–67
Sachdeva H, Dwivedi D, Khaturia S (2011) Aqua mediated facile synthesis of 2-(5/7-fluorinated-2-oxoindolin-3-ylidene)-N-(4-substituted phenyl) hydrazine carbothioamides. Res J Pharm Bio Chem Sci 2:213–219
Teng H (2012) Overview of the development of the fluoropolymer industry. Appl Sci 2:496–512
Wang J, Maria SR, Acena JL, Pozo C, Sorochinsky AE, Fustero S, Soloshonok VA, Liu H (2014) Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade (2001–2011) Chem. Rev. 114:2432–2506
Welch JT, Eswarakrishnan S (1991) Fluorine in bioorganic chemistry. Wiley, New York
Welch JT (1991) Selective fluorination in organic and bioorganic chemistry. American Chemical Society Washington DC
Erian AW (2001) Recent trends in the chemistry of fluorinated five and six-membered heterocycles. J Heterocycl Chem 38:793–808
Kirsch P (2004) Modern fluoroorganic chemistry: synthesis, reactivity and applications. Wiley, Weinheim
Uneyama K (2006) Organofluorine chemistry. Blackwell, Oxford
Regina GL, Coluccia A, Piscitelli F, Bergamini A, Sinistro A, Cavazza A (2007) Indolyl aryl sulfones as HIV-1 non-nucleoside reverse transcriptase inhibitors: role of two halogen atoms at the indole ring in developing new analogues with improved antiviral activity. J Med Chem 50:5034–5038
Ferro S, Luca LD, Barreca ML, Iraci N, Grazia SD, Christ F, Witvrouw M, Debyser Z, Chimirri A (2009) Docking studies on a new human immodeficiency virus integrase − Mg − DNA complex: phenyl ring exploration and synthesis of 1H-benzylindole derivatives through fluorine substitutions. J Med Chem 52:569–573
Filler R, Kobayashi Y, Yagupolskii (Eds.) L M (1993) Organofluorine compounds in medicinal chemistry and biomedical applications. Elsevier: Amsterdam
Isanbor C, O’Hagan D (2006) Fluorine in medicinal chemistry: a review of anti-cancer agents. J Fluorine Chem 27:303–319
Meanwell NA, Wallace OB, Fang H, Wang H, Deshpande M, Wang T, Yin Z, Zhang Z, Pearce BC, James J, Yeung KS, Qiu Z, Wright JJK, Yang Z, Zadjura L, Tweedie DL, Yeola S, Zhao F, Ranadive S, Robinson BA, Gong YF, Wang HGH, Blair WS, Shi PY, Colonno RJ, Lin PF (2009) Inhibitors of HIV-1 attachment. Part 2: an initial survey of indole substitution patterns. Bioorg Med Chem Lett 19:1977–1981
Haj Tehrani KME, Hashemi M, Hassan M, Kobarfard F, Mohebbi S (2016) Synthesis and antibacterial activity of Schiff bases of 5-substituted Isatins. Chin Chem Lett 27:221–225
Bjeldanes LF, Le HT, Firestone GL (2013) US Patent 2005/58600 (A1) 2005
Bari S, Mandi S, Ugale V, Rao V, Akena V (2015) Rational design and synthesis of benzothiazole-isatins for antimicrobial and cytotoxic activities. Indian J Chem 54B:418–429
Khan FA, Maalik A (2015) advances in pharmacology of isatin and its derivatives: a review. Tropical J Pharm Res 14:1937–1942
Lu RJ, Tucker JA, Zinevitch T, Kirichenko O, Konoplev V, Kuznetsova S, Sviridov S, Pickens J, Tandel S, Brahmachary E, Yang Y, Wang J, Freel S, Fisher S, Sullivan A, Zhou J, Stanfield OS, Greenberg M, Bolognesi D, Bray B, Koszalka B, Jeffs P, Khasanov A, Ma YA, Jeffries C, Liu C, Zhu T, Chucholowski A, Li R, Sexton C (2007) Design and synthesis of human immunodeficiency virus entry inhibitors: sulfonamide as an isostere for the α-ketoamide group. J Med Chem 50:6535–6544
Soubhye J, Aldib I, Elfving B, Gelbcke M, Furtmuller PG, Podrecca M, Conotte R, Colet JM, Rousseau A, Reye F, Sarakbi A, Vanhaeverbeek M, Kauffmann JM, Obinge C, Neve J, Prevost M, Boudjeltia KZ, Dufrasne F, Antwerpen PV (2013) Design, synthesis, and structure-activity relationship studies of novel 3-alkylindole derivatives as selective and highly potent myeloperoxidase inhibitors. J Med Chem 56:3943–3958
Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, El-Kouhen OF, Miller LN, Li L, Yao BB, Hsieh GC, Pai M, Zhu CZ, Chandran P, Meyer MD (2008) Indol-3-yl-tetramethylcyclopropyl Ketones: effects of indole ring substitution on CB2 cannabinoid receptor activity. J Med Chem 51:1904–1912
Acknowledgements
We are thankful to University Scientific Instrumentation Center, Karnatak University Dharwad, Karnataka, INDIA and SECAB A.R.S Inamdar College for Women, Vijayapur, Karnataka, INDIA for providing Instrumentation facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shaikh, I.N., Bagwan, U.F., Hunagund, S.M. et al. Cu-catalyzed Rapid Synthesis of Novel Fluorinated Indole Derivatives Under Microwave Irradiation. Chemistry Africa 1, 3–9 (2018). https://doi.org/10.1007/s42250-018-0013-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-018-0013-9