Abstract
Electronic contributions in electronic transitions and energetic data associated to the Mg-substitution in chlorophyll by three transition metals: chrom (Cr2+), iron (Fe2+) and nickel (Ni2+) have been studied theoretically using density functional theory and time dependent density functional theory (TD-DFT) methods. The binding energies are stronger than for Mg2+ in the case of all three cations especially in the case of Ni2+. The Mg-substitution process is found to be exergonic for all title elements in gas phase and in acetonitrile using both implicit and explicit models of solvation. The natural population analysis results, which estimated by natural bond orbital analysis, showed significant charge transfer from pheophytin ligand to the central cation. The UV–visible proprieties of the different substitution compounds have been studied using the TD-DFT method evidencing that substitution of Mg by Cr, Fe or Ni is associated to a blue shift of the Q-band for the three cations.


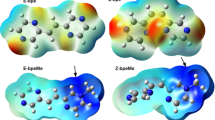
Adapted from ref. 32




Similar content being viewed by others
References
Blankenship RE (2002) Molecular Mechanisms of Photosynthesis. Blackwell Science Ltd., Oxford
Ke B (2001) Photosynthesis: photobiochemistry and photobiophysics. Kluwer Academic Publishers, Dordrecht
Whitmarsh J, Govindjee (1999) Dans concept in photobiology photosynthesis and photomorphogenesis. In: Singhal GS, Renger G, Sopory SK, Irragang KD, Govindjee (eds) The photosynthetic process. Norosa Publishers, New Delhi, pp 11–51
Callahan DL, Baker AJ, Kolev SD, Wedd AG (2006) Metal ion ligands in hyperaccumulating plants. J Biol Inorg Chem 11:2–12
Mustardy L, Garab G (2003) Trends Plant Sci 8:117–122
Shimoni E, Rav-Hon O, Ohad I, Brumfeld V, Reich Z (2005) Plant Cell 17:2580–2586
Mroz P, Bhaumik J, Dogutan DK, Aly Z, Kamal Z, Khalid L, Kee HL, Bocian DF, Holten D, Lindsey JS, Hamblin MR (2009) Cancer Lett 282:63–76
Amao Y, Yamada Y, Aoki K (2004) J Photochem Photobiol A 164:47–51
Wang X-F, Xiang J, Wang P, Koyama Y, Yanagida S, Wada Y, Hamada K, Sasaki S, Tamiaki H (2005) Chem Phys Lett 408:409–414
Wang X-F, Koyama Y, Kitao O, Wada Y, Sasaki S, Tamiaki H, Zhou H (2010) Biosens Bioelectron 25:1970–1976
Wang X-F, Kitao O, Hosono E, Zhou H, Sasaki S, Tamiaki H (2010) J Photochem Photobiol 210:145–152
Wang X-F, Tamiaki H, Wang L, Tamai N, Kitao O, Zhou H, Sasaki S (2010) Langmuir 26:6320–6327
Wang X-F, Kitao O (2012) Molecules 17:4484–4497
Ryan A, Senge MO (2015) Photochem Photobiol Sci 14:638–660
Hübner R, Astin KB, Herbert RJH (2010) J Environ Monit 12:1511–1514
Küpper H, Setlik I, Spiller M, Küpper FC, PráSil O (2002) J Phycol 38:429–441
Luna CM, González CA, Trippi VS (1994) Plant Cell Physiol 35:785–791
Bushnell TP, Bushnell D, Jagendorf AT (1993) Plant Physiol 103:585–591
Jegerschoeld C, Arellano JB, Schroeder WP, van Kan PJ, Baron M, Styring S (1995) Copper (II) inhibition of electron transfer through photosystem II studied by EPR spectroscopy. Biochemistry 34(39):12747–12754
Hsu BD, Lee JY (1988) Plant Physiol 87:116–119
Sas KN, Kovács L, Zsίros O et al (2006) J Biol Inorg Chem 11:725
Zvezdanovic J, Markovic D, Nikolic G (2007) J Serb Chem Soc 72:1053
Zvezdanovic J, Markovic D (2009) Russ J Phys Chem 38:1542
Boucher LJ, Katz JJ (1976) J Am Chem Soc 89:4703
Petrovic J, Nikolic G, Markovic D (2006) J Serb Chem Soc 71:501
Küpper H, Küpper F, Spiller M (1996) J Exp Bot 47:259
Küpper H, Lombi E, Zhao FJ, Wieshammer G, McGrath SP (2001) J Exp Bot 52:2291
Küpper H, Lombi E, Zhao FJ, McGrath SP (2000) Planta 212:75
De Filippis LF, Pallaghy CK (1976) Z Pflanzenphysiol 78:314
De Filippis LF (1979) Z Pflanzenphysiol 93:129
Bechaieb R, Ben Fredj A, Ben Akacha A, Gérard H (2016) N J Chem 40:4543
Bechaieb R, Ben Akacha A, Gérard H (2016) Chem Phys Lett 663:27–32
Gaussian 09, Revision D.01, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr. JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2013) Gaussian Inc., Wallingford
Becke AD (1993) Phys Chem 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B Condens Matter Mater Phys 37:785–789
Becke AD (1988) Phys Rev A At Mol Opt Phys 38:3098–3100
Scheidt WR, Reed CA (1981) Chem Rev 81:543–555
Crabtree RH (1988) The organometallic chemistry of the transition metals. John Wiley & Sons, Hoboken
Fredj AB, Lakhdar ZB, Ruiz-López MF (2008) Chem Commun 6:718–720
Ben Fredj A, Ruiz-López MF (2010) J Phys Chem B 114:681
Ben Fredj A, Ben Lakhdar Z, Ruiz-López MF (2009) Chem Phys Lett 472:243
Grimme S, Ehrlich S, Goerigk L (2011) J Comp Chem 32:1456
Orzeł Ł, van Eldik R, Fiedor L, Stochel G (2009) Eur J Inorg Chem 16:2393
Reed AE, Weinstock RB, Weinhold F (1985) J Chem Phys 83:735–746
Grotjohann I, Fromme P (2006) Photosynth Res 85:51
Dahlbom MG, Reimers R (2005) Mol Phys 103:1057
Sharma Y, Ganga P, Swapan KP (2011) J Phys Chem A 115:12298
Mulholland AR, Thordarson P, Mensforth EJ, Langford S (2012) J Org Biomol Chem 10:6045
Scheer H (éd) (1991) CRC Press, Boca Raton, p 3
Lerner DA, Balaceanu-Stolnici C, Weinberg J, Patron L (2015) Computational Chemistry 3:18–22
Singh RK, Verma SK, Sharma PD (2011) Int J Chem Tech Res 3:1571–1579
Vitnik VD, Vitnik ZJ, Banjac NR, Valentic NV, Uscumlic GS, Juranic IO (2014) Spectrochim Acta Part A Mol Biomol Spectrosc 117:42–53
Prasad MVS, Sri NU, Veeraiah A, Veeraiah V, Chaitanya K (2013) J At Mol Sci 4:1–17
Veinardi S, Sparisoma V (2012) J Sci 44A(2):93–112
Sundholm D (1999) Chem Phys Lett 302:480–484
Sundholm D (2000) Chem Phys Lett 317:545–552
Li Kai Yan (2010) Anna Pomogaeva, Feng Long Gu, Yuriko Aoki. Theor Chem Acc 125:511–520
Marcus Y (1991) J Chem Soc Faraday Trans 87:2995
Tomasi J, Mennucci B, Cammi R (2005) Chem Rev 105:2999
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic Supplementary Information (ESI) available: Cartesian coordinates and thermodynamic data for all optimized geometries and TD-DFT simulation. See DOI:10.1039/x0xx00000x.
Rights and permissions
About this article
Cite this article
Bechaieb, R., Lakhdar, Z.B. & Gérard, H. DFT and TD-DFT Studies of Mg-Substitution in Chlorophyll by Cr(II), Fe(II) and Ni(II). Chemistry Africa 1, 79–86 (2018). https://doi.org/10.1007/s42250-018-0003-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-018-0003-y