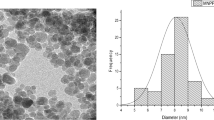
Abstract
The present study explored the green synthesis of a pineapple peel-iron manganese binary oxide magnetic nanocomposite (PP-IMBMN) and its effectiveness in adsorbing cationic methylene blue dye (MBD) from synthetically contaminated water. The PP-IMBMN adsorbent was characterized using SEM/EDAX, TEM, XRD, FTIR, VSM, and point of zero charge analyses. Batch experiments used varying operational parameters, including pH, initial dye concentration, adsorbent dosage, and temperature. Under optimum adsorption conditions, a removal efficiency of 92.68% was achieved. The Langmuir and Freundlich adsorption isotherm models provided good fits to the equilibrium data, albeit with a slightly superior correlation observed for the Langmuir model compared to the Freundlich model. Pseudo-second-order kinetics provides the best explanation for the adsorption data. The thermodynamic study suggested that the adsorption was feasible, endothermic, and accompanied by increased entropy. HCl was observed as the most effective desorbing agent, allowing the PP-IMBMN to be regenerated up to five times in the regeneration trials. Therefore, the PP-IMBMN) stands out as a compelling solution for removing MBD from industrial effluents, showcasing its novelty through remarkable adsorption affinity for dyes, ease of separation, affordability, and reusability.
Graphical Abstract







Similar content being viewed by others
Data availability
The relevant data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
R.M. Novais, G. Ascensão, D.M. Tobaldi, M.P. Seabra, J.A. Labrincha, Biomass fly ash geopolymer monoliths for effective methylene blue removal from wastewaters. J. Clean. Prod. 171, 783–794 (2018). https://doi.org/10.1016/j.jclepro.2017.10.078
R. Kant, Textile dyeing industry an environmental hazard. Nat. Sci. 04, 22–26 (2012). https://doi.org/10.4236/ns.2012.41004
H.B. Quesada, A.T.A. Baptista, L.F. Cusioli, D. Seibert, C. de Oliveira Bezerra, R. Bergamasco, Surface water pollution by pharmaceuticals and an alternative of removal by low-cost adsorbents: a review. Chemosphere. 222, 766–780 (2019). https://doi.org/10.1016/j.chemosphere.2019.02.009
L. Järup, L. Jarup, Hazards of heavy metal contamination. Br. Med. Bull. 68, 167–182 (2003). https://doi.org/10.1093/bmb/ldg032
B. Pradhan, S. Chand, S. Chand, P.R. Rout, S.K. Naik, Emerging groundwater contaminants: a comprehensive review on their health hazards and remediation technologies. Groundw. Sustain. Dev. 20, 100868 (2023). https://doi.org/10.1016/j.gsd.2022.100868
N. Morin-Crini, E. Lichtfouse, G. Liu, V. Balaram, A.R.L. Ribeiro, Z. Lu, F. Stock, E. Carmona, M.R. Teixeira, L.A. Picos-Corrales, J.C. Moreno-Piraján, L. Giraldo, C. Li, A. Pandey, D. Hocquet, G. Torri, G. Crini, Worldwide cases of water pollution by emerging contaminants: a review. Environ. Chem. Lett. 20, 2311–2338 (2022). https://doi.org/10.1007/s10311-022-01447-4
Y. Liu, P. Wang, B. Gojenko, J. Yu, L. Wei, D. Luo, T. Xiao, A review of water pollution arising from agriculture and mining activities in Central Asia: facts, causes and effects. Environ. Pollut. 291, 118209 (2021). https://doi.org/10.1016/j.envpol.2021.118209
A. Nasar, Utilization of tea wastes for the removal of toxic dyes from polluted water—a review. Biomass Convers. Biorefinery. 13, 1399–1415 (2023). https://doi.org/10.1007/s13399-020-01205-y
I. Nassri, S. Khattabi Rifi, F. Sayerh, S. Souabi, Occurrence, pollution sources, and mitigation prospects of Antibiotics, anti-inflammatories, and endocrine disruptors in the aquatic environment. Environ. Nanotechnol. Monit. Manag. 20, 100878 (2023). https://doi.org/10.1016/j.enmm.2023.100878
N.A. Qamruzzaman, Kinetics of metribuzin degradation by colloidal manganese dioxide in absence and presence of surfactants. Chem. Pap. 68, 65–73 (2014). https://doi.org/10.2478/s11696-013-0424-7
N.A. Qamruzzaman, Treatment of acetamiprid insecticide from artificially contaminated water by colloidal manganese dioxide in the absence and presence of surfactants. RSC Adv. 4, 62844–62850 (2014). https://doi.org/10.1039/c4ra09685a
J. Gregory, R. V. Dhond, Wastewater treatment by ion exchange. Water Res. 6, (1972). https://doi.org/10.1016/0043-1354(72)90183-2
S.A. Butani, S.J. Mane, Coagulation / flocculation process for cationic, anionic dye removal using water treatment residuals – a review. Int. J. Sci. Technol. Manag. 6, 121–125 (2017)
A.K. Verma, R.R. Dash, P. Bhunia, A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manage. 93, 154–168 (2012). https://doi.org/10.1016/j.jenvman.2011.09.012
O. Amuda, I. Amoo, Coagulation/flocculation process and sludge conditioning in beverage industrial wastewater treatment. J. Hazard. Mater. 141, 778–783 (2007). https://doi.org/10.1016/j.jhazmat.2006.07.044
L.F. Greenlee, D.F. Lawler, B.D. Freeman, B. Marrot, P. Moulin, Reverse osmosis desalination: water sources, technology, and today’s challenges. Water Res. 43, 2317–2348 (2009). https://doi.org/10.1016/j.watres.2009.03.010
A. Cassano, R. Molinari, M. Romano, E. Drioli, Treatment of aqueous effluents of the leather industry by membrane processes. J. Memb. Sci. 181, 111–126 (2001). https://doi.org/10.1016/S0376-7388(00)00399-9
D. Bahnemann, Photocatalytic water treatment: solar energy applications. Sol. Energy 77, 445–459 (2004). https://doi.org/10.1016/j.solener.2004.03.031
S. Al-Amshawee, M.Y.B.M. Yunus, A.A.M. Azoddein, D.G. Hassell, I.H. Dakhil, H.A. Hasan, Electrodialysis desalination for water and wastewater: a review. Chem. Eng. J. 380, 122231 (2020). https://doi.org/10.1016/j.cej.2019.122231
E. Korngold, K. Kock, H. Strathmann, Electrodialysis in advanced waste water treatment. Desalination 24, 129–139 (1977). https://doi.org/10.1016/S0011-9164(00)88079-0
S.H. Lin, C.M. Lin, Treatment of textile waste effluents by ozonation and chemical coagulation. Water Res. 27, 1743–1748 (1993). https://doi.org/10.1016/0043-1354(93)90112-U
S. Khamparia, D.K. Jaspal, Adsorption in combination with ozonation for the treatment of textile waste water: a critical review. Front. Environ. Sci. Eng. 11, 8 (2017). https://doi.org/10.1007/s11783-017-0899-5
S. Chakraborty, M.K. Purkait, S. DasGupta, S. De, J.K. Basu, Nanofiltration of textile plant effluent for color removal and reduction in COD. Sep. Purif. Technol. 31, 141–151 (2003). https://doi.org/10.1016/S1383-5866(02)00177-6
M.T. Yagub, T.K. Sen, S. Afroze, H.M. Ang, Dye and its removal from aqueous solution by adsorption: a review. Adv. Colloid Interface Sci. 209, 172–184 (2014). https://doi.org/10.1016/j.cis.2014.04.002
H.K. Rajendran, M. Das, R. Chandrasekar, M.A. Deen, B. Murugan, S. Narayanasamy, L. Sahoo, UiO-66 octahedrons for adsorptive removal of direct blue-6: process optimization, interaction mechanism, and phytotoxicity assessment. Environ. Sci. Pollut. Res. 30, 114264–114282 (2023). https://doi.org/10.1007/s11356-023-30296-z
R. Chandrasekar, H.K. Rajendran, V. Priyan, V.S. Narayanasamy, Valorization of sawdust by mineral acid assisted hydrothermal carbonization for the adsorptive removal of bisphenol A: a greener approach. Chemosphere. 303, 135171 (2022). https://doi.org/10.1016/j.chemosphere.2022.135171
S. Rajoriya, V. Kumar, A. Singh, M. Nigam, K. Roy, Current Research in Green and Sustainable Chemistry Adsorption of methyl red dye from aqueous solution onto eggshell waste material : kinetics, isotherms and thermodynamic studies. Curr. Res. Green Sustain. Chem. 4, 100180 (2021). https://doi.org/10.1016/j.crgsc.2021.100180
W.T. Tsai, J.M. Yang, C.W. Lai, Y.H. Cheng, C.C. Lin, C.W. Yeh, Characterization and adsorption properties of eggshells and eggshell membrane. Bioresour. Technol. 97, 488–493 (2006). https://doi.org/10.1016/j.biortech.2005.02.050
M.T. Yagub, T.K. Sen, H.M. Ang, Equilibrium, kinetics, and thermodynamics of methylene blue adsorption by pine tree leaves. Water Air Soil Pollut. 223, 5267–5282 (2012). https://doi.org/10.1007/s11270-012-1277-3
H. Tahir, M. Sultan, N. Akhtar, U. Hameed, T. Abid, Application of natural and modified sugar cane bagasse for the removal of dye from aqueous solution. J. Saudi Chem. Soc. 20, S115–S121 (2016). https://doi.org/10.1016/j.jscs.2012.09.007
A.S. Sartape, A.M. Mandhare, V.V. Jadhav, P.D. Raut, M.A. Anuse, S.S. Kolekar, Removal of malachite green dye from aqueous solution with adsorption technique using Limonia acidissima (wood apple) shell as low cost adsorbent. Arab. J. Chem. 10, S3229–S3238 (2017). https://doi.org/10.1016/j.arabjc.2013.12.019
M.K. Uddin, A. Nasar, Decolorization of basic dyes solution by utilizing fruit seed powder. KSCE J. Civ. Eng. 24, 345–355 (2020). https://doi.org/10.1007/s12205-020-0523-2
S. Wong, H.H. Tumari, N. Ngadi, N.B. Mohamed, O. Hassan, R. Mat, N.A. Saidina Amin, Adsorption of anionic dyes on spent tea leaves modified with polyethyleneimine (PEI-STL). J. Clean. Prod. 206, 394–406 (2019). https://doi.org/10.1016/j.jclepro.2018.09.201
R.J. Khan, A.N.S. Saqib, R. Farooq, R. Khan, M. Siddique, Removal of congo red from aqueous solutions by spent black tea as adsorbent. J. Water Chem. Technol. 40, 206–212 (2018). https://doi.org/10.3103/S1063455X18040057
M. El-Azazy, A.S. El-Shafie, A.A. Issa, M. Al-Sulaiti, J. Al-Yafie, B. Shomar, K. Al-Saad, Potato Peels as an adsorbent for heavy metals from aqueous solutions: eco-structuring of a green adsorbent operating Plackett-Burman design. J. Chem. 2019, 1–14 (2019). https://doi.org/10.1155/2019/4926240
K. Ben Jeddou, F. Bouaziz, F. Ben Taheur, O. Nouri-Ellouz, R. Ellouz-Ghorbel, S. Ellouz-Chaabouni, Adsorptive removal of direct red 80 and methylene blue from aqueous solution by potato peels: a comparison of anionic and cationic dyes. Water Sci. Technol. 83, 1384–1398 (2021). https://doi.org/10.2166/wst.2021.075
G.K. Cheruiyot, W.C. Wanyonyi, J.J. Kiplimo, E.N. Maina, Adsorption of toxic crystal violet dye using coffee husks: equilibrium, kinetics and thermodynamics study. Sci. African. 5, e00116 (2019). https://doi.org/10.1016/j.sciaf.2019.e00116
A.S. Franca, L.S. Oliveira, M.E. Ferreira, Kinetics and equilibrium studies of methylene blue adsorption by spent coffee grounds. Desalination 249, 267–272 (2009). https://doi.org/10.1016/j.desal.2008.11.017
F.A. Pavan, A.C. Mazzocato, Y. Gushikem, Removal of methylene blue dye from aqueous solutions by adsorption using yellow passion fruit peel as adsorbent. Bioresour. Technol. 99, 3162–3165 (2008). https://doi.org/10.1016/j.biortech.2007.05.067
D. Sidiras, F. Batzias, E. Schroeder, R. Ranjan, M. Tsapatsis, Dye adsorption on autohydrolyzed pine sawdust in batch and fixed-bed systems. Chem. Eng. J. 171, 883–896 (2011). https://doi.org/10.1016/j.cej.2011.04.029
U.B.B. Deshannavar, G.M.M. Ratnamala, P.B.B. Kalburgi, M. El-Harbawi, A. Agarwal, M. Shet, M. Teli, P. Bhandare, Optimization, kinetic and equilibrium studies of disperse yellow 22 dye removal from aqueous solutions using Malaysian teak wood sawdust as adsorbent. Indian Chem. Eng. 58, 12–28 (2016). https://doi.org/10.1080/00194506.2014.987831
Z. Ahamad, A. Nasar, Utilization of Azadirachta indica sawdust as a potential adsorbent for the removal of crystal violet dye. Sustain. Chem. 4, 110–126 (2023). https://doi.org/10.3390/suschem4010009
F. Mashkoor, A. Nasar, C. Jeong, Magnetized chitosan nanocomposite as an effective adsorbent for the removal of methylene blue and malachite green dyes. Biomass Convers. Biorefinery. (2022). https://doi.org/10.1007/s13399-021-02282-3
Z. Ahamad, A. Nasar, Polypyrrole-decorated bentonite magnetic nanocomposite: a green approach for adsorption of anionic methyl orange and cationic crystal violet dyes from contaminated water. Environ. Res. 247, 118193 (2024). https://doi.org/10.1016/j.envres.2024.118193
Y. Yao, S. Miao, S. Liu, L.P. Ma, H. Sun, S. Wang, Synthesis, characterization, and adsorption properties of magnetic Fe3O4@graphene nanocomposite. Chem. Eng. J. 184, 326–332 (2012). https://doi.org/10.1016/j.cej.2011.12.017
Q. Liu, L.-B. Zhong, Q.-B. Zhao, C. Frear, Y.-M. Zheng, Synthesis of Fe3O4/polyacrylonitrile composite electrospun nanofiber mat for effective adsorption of tetracycline. ACS Appl. Mater. Interfaces 7, 14573–14583 (2015). https://doi.org/10.1021/acsami.5b04598
N. Kataria, V.K. Garg, Green synthesis of Fe3O4 nanoparticles loaded sawdust carbon for cadmium (II) removal from water: regeneration and mechanism. Chemosphere 208, 818–828 (2018). https://doi.org/10.1016/j.chemosphere.2018.06.022
G. Zhang, X. Xu, Q. Ji, R. Liu, H. Liu, J. Qu, J. Li, Porous nanobimetallic Fe-Mn cubes with high valent Mn and highly efficient removal of arsenic(III). ACS Appl. Mater. Interfaces 9, 14868–14877 (2017). https://doi.org/10.1021/acsami.7b02127
K. Lu, T. Wang, L. Zhai, W. Wu, S. Dong, S. Gao, L. Mao, Adsorption behavior and mechanism of Fe-Mn binary oxide nanoparticles : adsorption of methylene blue. J. Colloid Interface Sci. (2018). https://doi.org/10.1016/j.jcis.2018.12.094
G. Yin, X. Chen, B. Sarkar, N.S. Bolan, T. Wei, H. Zhou, H. Wang, Co-adsorption mechanisms of Cd(II) and As(III) by an Fe-Mn binary oxide biochar in aqueous solution. Chem. Eng. J. 466, 143199 (2023). https://doi.org/10.1016/j.cej.2023.143199
G. Zhao, J. Li, X. Niu, K. Tang, S. Wang, W. Zhu, X. Ma, M. Ru, Y. Yang, Facile synthesis of Mn-doped Fe2O3 nanostructures: enhanced CO catalytic performance induced by manganese doping. New J. Chem. 40, 3491–3498 (2016). https://doi.org/10.1039/c5nj03694a
S. Zhang, X. Fan, J. Xue, A novel magnetic manganese oxide halloysite composite by one-pot synthesis for the removal of methylene blue from aqueous solution. J. Alloys Compd. 930, 167050 (2023). https://doi.org/10.1016/j.jallcom.2022.167050
M.A. El-Ghobashy, I.A. Salem, W.M. El-Dahrawy, M.A. Salem, Fabrication of α-MnO2/Fe-Mn binary oxide nanocomposite as an efficient adsorbent for the removal of methylene blue from wastewater. J. Mol. Struct. 1272, 134118 (2023). https://doi.org/10.1016/j.molstruc.2022.134118
C. Yang, T. Ju, X. Wang, Y. Ji, C. Yang, H. Lv, The preparation of a novel iron / manganese binary oxide for the efficient removal of hexavalent chromium [Cr ( VI )] from aqueous solutions. RSC Adv. 10, 10612–10623 (2020). https://doi.org/10.1039/C9RA10558A
T. Huang, D. Song, C. Yang, S. Zhang, Nonthermal plasma-irradiated polyvalent ferromanganese binary hydro(oxide) for the removal of uranyl ions from wastewater. Environ. Res. 217, 114911 (2023). https://doi.org/10.1016/j.envres.2022.114911
J. Lu, F. Zhang, Novel Fe–Mn oxide/zeolite composite material for rapid removal of toxic copper ions from aqueous solutions. J. Clean. Prod. 397, 136496 (2023). https://doi.org/10.1016/j.jclepro.2023.136496
S. Hmamouchi, A. El Yacoubi, B.C. El Idrissi, Using egg ovalbumin to synthesize pure α-Fe2O3 and cobalt doped α-Fe2O3: structural, morphological, optical and photocatalytic properties. Heliyon. 8, e08953 (2022). https://doi.org/10.1016/j.heliyon.2022.e08953
M. Rahmayanti, A. Nurul Syakina, I. Fatimah, T. Sulistyaningsih, Green synthesis of magnetite nanoparticles using peel extract of jengkol (Archidendron pauciflorum) for methylene blue adsorption from aqueous media. Chem. Phys. Lett. 803, 139834 (2022). https://doi.org/10.1016/j.cplett.2022.139834
N. Faaizatunnisa, R. Ediati, H. Fansuri, H. Juwono, S. Suprapto, A.R.P. Hidayat, L.L. Zulfa, Facile green synthesis of core–shell magnetic MOF composites (Fe3O4@SiO2@HKUST-1) for enhanced adsorption capacity of methylene blue. Nano-Struct. Nano-Objects. 34, 100968 (2023). https://doi.org/10.1016/j.nanoso.2023.100968
J. Xi, R. Zhang, L. Ye, X. Du, X. Lu, Multi-step preparation of Fe and Si modified biochar derived from waterworks sludge towards methylene blue adsorption. J. Environ. Manage. 304, 114297 (2022). https://doi.org/10.1016/j.jenvman.2021.114297
K.Y. Foo, B.H. Hameed, Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 156, 2–10 (2010). https://doi.org/10.1016/j.cej.2009.09.013
Author information
Authors and Affiliations
Contributions
First author: sample preparation, experimental studies, calculations, figures, graphical designing, original draft; second (corresponding) author: supervision, management, review, editing, finalization. All authors have discussed the final manuscript and decided to submit it.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmed, M., Nasar, A. Advancements in water purification: green synthesis of pineapple peel-derived iron manganese binary oxide magnetic nanocomposites for efficient methylene blue adsorption. emergent mater. (2024). https://doi.org/10.1007/s42247-024-00693-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42247-024-00693-2