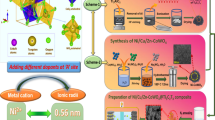
Abstract
Vast research activities were employed to identify efficient cathode materials for supercapacitor. In this present work, nickel aluminium–layered double hydroxide (NiAl LDH)–decorated vanadium pentoxide (NiAl LDH/V2O5) was prepared via a single-step hydrothermal technique. Here, the systematic analysis has been carried out using various analytical techniques to study phase purity, functional group, morphology, chemical state, and electrochemical properties of the prepared samples. BET results revealed that NiAl LDH/V2O5 hybrid composite displays an optimized surface area of about 277.677 m2 g−1 and pore radius of about 1.617 nm. The electrochemical performance for NiAl LDH/V2O5 composite is high among the prepared samples with a specific capacitance of 1169 Fg−1 at 1 Ag−1 and cyclic stability 91% for 5000 charge-discharge cycles. Furthermore, the asymmetric device was fabricated with rGO as anode and NiAl LDH/V2O5 as cathode, the fabricated device delivered the specific capacitance of 129 Fg−1 at 2 Ag−1, and a better specific power of 4654 W kg−1 at a specific energy of 43.1 W h kg−1 with 93% stability after 10,000 cycles. The better performances of NiAl LDH/V2O5 composite compared to other materials might be due to the enhanced surface area which enhanced the electronic conductivity among the materials by rendering high redox-rich active sites and rapid ionic mobility.
Graphical abstract








Similar content being viewed by others
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
A. Yu, A. Davies, Z. Chen, Electrochemical supercapacitors. Electrochem. Technol. Energy Storage Convers. 1, 317–382 (2012). https://doi.org/10.1002/9783527639496.ch8
E.B. Conway, Electrochemical supercapacitors scientific fundamentals and technological applications. Adv. Lithium-Ion Batt. 481–505 (1999). https://doi.org/10.1007/0-306-47508-1_17
B.E. Conway, V. Birss, J. Wojtowicz, The role and utilization of pseudocapacitance for energy storage by supercapacitors. J. Power Sources 66, 1–14 (1997). https://doi.org/10.1016/S0378-7753(96)02474-3
Y. Gogotsi, P. Simon, Materials for electrochemical capacitors. Nat. Mater. 7, 845–854 (2008)
V. Augustyn, P. Simon, B. Dunn, Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 7, 1597–1614 (2014). https://doi.org/10.1039/c3ee44164d
M. Priyadharshini, M. Sandhiya, M. Sathish, et al., Surfactant-dependant self organisation of nickel pyrophosphate for electrochemical supercapacitors. J. Mater. Sci. Mater. Electron. (2021). https://doi.org/10.1007/s10854-021-07261-y
P. Matheswaran, P. Karuppiah, S.M. Chen, et al., Fabrication of g-C3N4 nanomesh-anchored amorphous NiCoP2O7: tuned cycling life and the dynamic behavior of a hybrid capacitor. ACS Omega. 3, 18694–18704 (2018). https://doi.org/10.1021/acsomega.8b02635
Y.P. Gao, C.N. Sisk, L.J. Hope-Weeks, A sol-gel route to synthesize monolithic zinc oxide aerogels. Chem. Mater. 19, 6007–6011 (2007). https://doi.org/10.1021/cm0718419
A.V. Nikam, B.L.V. Prasad, A.A. Kulkarni, Wet chemical synthesis of metal oxide nanoparticles: a review. CrystEngComm. 20, 5091–5107 (2018). https://doi.org/10.1039/C8CE00487K
T. Cottineau, M. Toupin, T. Delahaye, et al., Nanostructured transition metal oxides for aqueous hybrid electrochemical supercapacitors. Appl. Phys. A Mater. Sci. Process. 82, 599–606 (2006). https://doi.org/10.1007/s00339-005-3401-3
X. Gong, N.I. Beedri, M.F.A. Taleb, et al., An overview of bi-layered niobium pentoxide (Nb2O5)-based photoanodes for dye-sensitized solar cells. Adv. Compos. Hybrid Mater. 6, 213 (2023). https://doi.org/10.1007/s42114-023-00791-5
Y. Kuthati, R.K. Kankala, C.-H. Lee, Layered double hydroxide nanoparticles for biomedical applications: current status and recent prospects. Appl. Clay Sci. 112–113, 100–116 (2015). https://doi.org/10.1016/j.clay.2015.04.018
A. Udayakumar, P. Dhandapani, S. Ramasamy, S. Angaiah, Layered double hydroxide (LDH) – MXene Nanocomposite for electrocatalytic water splitting: current status and perspective. ES Energy Environ. 20, 902 (2023). https://doi.org/10.30919/esee902
Z.Z. Yang, J.J. Wei, G.M. Zeng, et al., A review on strategies to LDH-based materials to improve adsorption capacity and photoreduction efficiency for CO2. Coord. Chem. Rev. 386, 154–182 (2019). https://doi.org/10.1016/j.ccr.2019.01.018
D. Su, Z. Tang, J. Xie, et al., Co, Mn-LDH nanoneedle arrays grown on Ni foam for high performance supercapacitors. Appl. Surf. Sci. 469, 487–494 (2019). https://doi.org/10.1016/j.apsusc.2018.10.276
W. Qiang, O. Dermot, Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 112, 4124–4155 (2012)
X. Ge, C. Gu, Z. Yin, et al., Periodic stacking of 2D charged sheets: self-assembled superlattice of Ni-Al layered double hydroxide (LDH) and reduced graphene oxide. Nano. Energy. 20, 185–193 (2016). https://doi.org/10.1016/j.nanoen.2015.12.020
L. Zhang, K.N. Hui, K.S. Hui, et al., Role of graphene on hierarchical flower-like NiAl layered double hydroxide-nickel foam-graphene as binder-free electrode for high-rate hybrid supercapacitor. Int. J. Hydrog. Energy. 41, 9443–9453 (2016). https://doi.org/10.1016/j.ijhydene.2016.04.050
L. Li, K.S. Hui, K.N. Hui, et al., High-performance solid-state flexible supercapacitor based on reduced graphene oxide/hierarchical core-shell Ag nanowire@NiAl layered double hydroxide film electrode. Chem. Eng. J. 348, 338–349 (2018). https://doi.org/10.1016/j.cej.2018.04.164
W. Zheng, S. Sun, Y. Xu, et al., Sulfidation of hierarchical NiAl−LDH/Ni−MOF composite for high-performance supercapacitor. ChemElectroChem. 6, 3375–3382 (2019). https://doi.org/10.1002/celc.201900687
B. Saravanakumar, K.K. Purushothaman, G. Muralidharan, High performance supercapacitor based on carbon coated V2O5 nanorods. J. Electroanal. Chem. 758, 111–116 (2015). https://doi.org/10.1016/j.jelechem.2015.10.031
Y. Zhang, Y. Wang, Z. Xiong, et al., V2O5 Nanowire composite paper as a high-performance lithium-ion battery cathode. ACS Omega. 2, 793–799 (2017). https://doi.org/10.1021/acsomega.7b00037
D.J. Ahirrao, K. Mohanapriya, N. Jha, V2O5 nanowires-graphene composite as an outstanding electrode material for high electrochemical performance and long-cycle-life supercapacitor. Mater. Res. Bull. 108, 73–82 (2018). https://doi.org/10.1016/j.materresbull.2018.08.028
D. Majumdar, M. Mandal, S.K. Bhattacharya, V2O5 and its carbon-based nanocomposites for supercapacitor applications. ChemElectroChem 6, 1623–1648 (2019). https://doi.org/10.1002/celc.201801761
W. Zheng, S. Sun, Y. Xu, et al., Facile synthesis of NiAl-LDH/MnO2 and NiFe-LDH/MnO2 composites for high-performance asymmetric supercapacitors. J. Alloys Compd. 768, 240–248 (2018). https://doi.org/10.1016/j.jallcom.2018.07.168
Y. Wei, X. Zhang, X. Wu, et al., Carbon quantum dots/Ni-Al layered double hydroxide composite for high-performance supercapacitors. RSC Adv. 6, 39317–39322 (2016). https://doi.org/10.1039/c6ra02730j
W. Wang, N. Zhang, Z. Shi, et al., Preparation of Ni-Al layered double hydroxide hollow microspheres for supercapacitor electrode. Chem. Eng. J. 338, 55–61 (2018). https://doi.org/10.1016/j.cej.2018.01.024
P. Matheswaran, P. Thangavelu, B. Ganapathi, Controlled approach on spinel lithium manganese oxide as possible cathode for high-performance Li-ion supercapacitors. Mater. Technol. (2019). https://doi.org/10.1080/10667857.2019.1615275
S.J. Marje, P.K. Katkar, S.S. Pujari, et al., Regulated micro-leaf like nickel pyrophosphate as a cathode electrode for asymmetric supercapacitor. Synth. Met. 259, 116224 (2020). https://doi.org/10.1016/j.synthmet.2019.116224
M. Priyadharshini, T. Pazhanivel, G. Bharathi, Carbon quantum dot incorporated nickel pyrophosphate as alternate cathode for supercapacitors. ChemistrySelect. 5, 2643–2652 (2020). https://doi.org/10.1002/slct.201904334
P. Matheswaran, P. Karuppiah, S.M. Chen, P. Thangavelu, A binder-free Ni2P2O7/Co2P2O7 nanograss array as an efficient cathode for supercapacitors. New J. Chem. 44, 13131–13140 (2020). https://doi.org/10.1039/d0nj00890g
X. Liu, J. Xu, L. Ma, et al., Nano-flower S-scheme heterojunction NiAl-LDH/MoS2for enhancing photocatalytic hydrogen production. New J. Chem. 46, 228–238 (2022). https://doi.org/10.1039/d1nj04728k
N.R. Palapa, T. Taher, R. Mohadi, et al., NiAl-layered double hydroxide intercalated with Keggin polyoxometalate as adsorbent of malachite green: kinetic and equilibrium studies. Chem. Eng. Commun. 209, 684–695 (2022). https://doi.org/10.1080/00986445.2021.1895773
G. Yang, T. Takei, S. Yanagida, N. Kumada, Synthesis and electrochemical properties of CoAl, NiAl, CoFe and NiFe layered double hydroxide films. J. Ion Exch. 29, 131–135 (2018). https://doi.org/10.5182/jaie.29.131
X. Li, M. Fortunato, A.M. Cardinale, et al., Electrochemical study on nickel aluminum layered double hydroxides as high-performance electrode material for lithium-ion batteries based on sodium alginate binder. J. Solid State Electrochem. 26, 49–61 (2022). https://doi.org/10.1007/s10008-021-05011-y
X. Li, L. Yu, G. Wang, et al., Hierarchical NiAl LDH nanotubes constructed via atomic layer deposition assisted method for high performance supercapacitors. Electrochim. Acta. 255, 15–22 (2017). https://doi.org/10.1016/j.electacta.2017.09.155
P. Matheswaran, P. Karuppiah, P. Thangavelu, Contribution of different charge storage mechanisms in cobalt pyrophosphate–based supercapattery. Ionics (Kiel). (2021). https://doi.org/10.1007/s11581-021-03908-2
F. Yu, Z. Liu, R. Zhou, et al., Pseudocapacitance contribution in boron-doped graphite sheets for anion storage enables high-performance sodium-ion capacitors. Mater Horiz. 5, 529–535 (2018). https://doi.org/10.1039/c8mh00156a
N.I. Chandrasekaran, M. Kumari, H. Muthukumar, M. Matheswaran, Strategy for multifunctional hollow shelled triple oxide Mn-Cu-Al nanocomposite synthesis via microwave-assisted technique. ACS Sustain. Chem. Eng. 6, 1009–1021 (2018). https://doi.org/10.1021/acssuschemeng.7b03339
J. Kumar, R.R. Neiber, Z. Abbas, et al., Hierarchical NiMn-LDH hollow spheres as a promising pseudocapacitive electrode for supercapacitor application. Micromachines (Basel). 14 (2023). https://doi.org/10.3390/mi14020487
J. Fang, M. Li, Q. Li, et al., Microwave-assisted synthesis of CoAl-layered double hydroxide/graphene oxide composite and its application in supercapacitors. Electrochim. Acta. 85, 248–255 (2012). https://doi.org/10.1016/j.electacta.2012.08.078
B. Wang, Q. Liu, Z. Qian, et al., Two steps in situ structure fabrication of Ni–Al layered double hydroxide on Ni foam and its electrochemical performance for supercapacitors. J. Power Sources. 246, 747–753 (2014). https://doi.org/10.1016/j.jpowsour.2013.08.035
H. Sim, C. Jo, T. Yu, et al., Reverse micelle synthesis of colloidal nickel–manganese layered double hydroxide nanosheets and their pseudocapacitive properties. Chemistry – A. Eur. J. Dermatol. 20, 14880–14884 (2014). https://doi.org/10.1002/chem.201403991
L. Li, R. Li, S. Gai, et al., Facile fabrication and electrochemical performance of flower-like Fe 3 O 4 @C@layered double hydroxide (LDH) composite. J. Mater. Chem. A 2, 8758–8765 (2014). https://doi.org/10.1039/C4TA01186D
L. Su, C. Ma, T. Hou, W. Han, Selective synthesis and capacitive characteristics of CoNiAl three-component layered double hydroxide platelets. RSC Adv. 3, 19807 (2013). https://doi.org/10.1039/c3ra42567c
J. Yang, C. Yu, X. Fan, et al., Facile fabrication of MWCNT-doped NiCoAl-layered double hydroxide nanosheets with enhanced electrochemical performances. J. Mater. Chem. A 1, 1963–1968 (2013). https://doi.org/10.1039/C2TA00832G
M.Z. Iqbal, M.M. Faisal, S.R. Ali, A.M. Afzal, Hydrothermally synthesized zinc phosphate-rGO composites for supercapattery devices. J. Electroanal. Chem. 871, 114299 (2020). https://doi.org/10.1016/j.jelechem.2020.114299
P. Li, Y. Jiao, S. Yao, et al., Dual role of nickel foam in NiCoAl-LDH ensuring high-performance for asymmetric supercapacitors. New J. Chem. 43, 3139–3145 (2019). https://doi.org/10.1039/C8NJ05447A
X. Bai, Q. Liu, J. Liu, et al., Hierarchical Co3O4 @Ni(OH) 2 core-shell nanosheet arrays for isolated all-solid state supercapacitor electrodes with superior electrochemical performance. Chem. Eng. J. 315, 35–45 (2017). https://doi.org/10.1016/j.cej.2017.01.010
X. Wu, L. Jiang, C. Long, et al., Dual support system ensuring porous Co–Al hydroxide nanosheets with ultrahigh rate performance and high energy density for supercapacitors. Adv. Funct. Mater. 25, 1648–1655 (2015). https://doi.org/10.1002/adfm.201404142
X. Bai, Q. Liu, H. Zhang, et al., Nickel-cobalt layered double hydroxide nanowires on three dimensional graphene nickel foam for high performance asymmetric supercapacitors. Electrochim. Acta. 215, 492–499 (2016). https://doi.org/10.1016/j.electacta.2016.08.134
Y. Cheng, H. Zhang, C.V. Varanasi, J. Liu, Improving the performance of cobalt–nickel hydroxide-based self-supporting electrodes for supercapacitors using accumulative approaches. Energy Environ. Sci. 6, 3314 (2013). https://doi.org/10.1039/c3ee41143e
J. Han, Y. Dou, J. Zhao, et al., Flexible CoAl LDH@PEDOT core/shell nanoplatelet array for high-performance energy storage. Small. 9, 98–106 (2013). https://doi.org/10.1002/smll.201201336
F.B.M. Ahmed, D. Khalafallah, M. Zhi, Z. Hong, Porous nanoframes of sulfurized NiAl layered double hydroxides and ternary bismuth cerium sulfide for supercapacitor electrodes. Adv. Compos. Hybrid Mater. 5, 2500–2514 (2022). https://doi.org/10.1007/s42114-022-00496-1
F. Wang, S. Sun, Y. Xu, et al., High performance asymmetric supercapacitor based on cobalt nickle iron-layered double hydroxide/carbon nanofibres and activated carbon. Sci. Rep. 7, 4707 (2017). https://doi.org/10.1038/s41598-017-04807-1
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hariprasath, K., Priyadharshini, M., Balaji, P. et al. V2O5-assembled NiAl-layered double hydroxide as high-performance electrode for asymmetric supercapacitor. emergent mater. (2024). https://doi.org/10.1007/s42247-024-00680-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42247-024-00680-7