Abstract
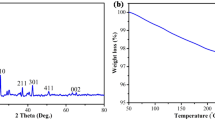
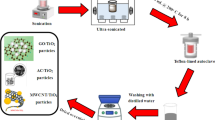
The release of toxic hydrogen sulfide gas from subsurface oil wells during oil and gas drilling operations leads to serious health and safety problems for both the drilling structures and working personnel. The implementation of controls and mitigation techniques is vital to abating the high health and economic risks associated with H2S exposure. The effective removal of H2S gas in-situ during drilling operations is key to limiting these risks. On the other hand, utilizing waste cooking oils for formulating oil-based fluids will transfer these waste oils into valuable commodities, and will make the drilling operations greener and more sustainable. Thus, waste cooking oil was utilized in this study to formulate oil-based drilling mud (OBM). The mud contained zeolitic imidazolate framework-8 (ZIF-8) NPs as an emerging material with H2S scavenging capability. The synthesized ZIF-8 NPs were characterized by XRD, FT-IR, TGA, and the nitrogen (N2) sorption isotherm. The influence of the ZIF-8 NPs on the H2S scavenging capacity and viscosity of this green and sustainable OBM were investigated and compared to the base mud without ZIF-8 NPs. The incorporation of ZIF-8 NPs significantly enhanced the H2S scavenging capacity of the drilling fluid. The breakthrough and saturation capacities of the drilling fluid were enhanced by 150 and 245% with the addition of ZIF-8 NPs. The plastic and apparent viscosities exhibited similar behavior and were not compromised by the incorporation of ZIF-8 NPs.











Similar content being viewed by others
Data availability
Data will be made available upon reasonable requests.
References
M. Murtaza, S.A. Alarifi, A. Abozuhairah, M. Mahmoud, S.A. Onaizi, M. Al-Ajmi, Optimum selection of H2S scavenger in light-weight and heavy-weight water-based drilling fluids. ACS Omega 6, 24919–24930 (2021). https://doi.org/10.1021/acsomega.1c03792
E. Sunde, H. Olsen, Removal of H2S in Drilling Mud. U.S. Patent 6,365,053 B1, (2002).
J. Buller, J.F. Carpenter, H2S Scavengers for Non-Aqueous Systems, Proceedings - SPE International Symposium on Oilfield Chemistry. 575–579 (2005). https://doi.org/10.2118/93353-MS.
R.A. Marriott, P. Pirzadeh, J.J. Marrugo-Hernandez, S. Raval, Hydrogen sulfide formation in oil and gas. Can. J. Chem. 94, 406–413 (2016). https://doi.org/10.1139/cjc-2015-0425
D.T. Oakes, Method for reducing hydrogen sulfide concentrations in well fluids, U.S. Patent 4,473,115 A, (1984).
H. Dembicki, Jr., Interpreting crude oil and natural gas data, In: Practical petroleum geochemistry for exploration and production, Elsevier, 2017: pp. 135–188. https://doi.org/10.1016/B978-0-12-803350-0.00004-0
S.A. Onaizi, R. Shawabkeh, Z.O. Malaibari, N.S. Abu-ghander, Method of sweetening hydrocarbon gas from hydrogen sulfide, (2020). https://patents.google.com/patent/US20200197865A1/en. Accessed 27 Mar 2023
L.E. Fletcher, Potential explosive hazards from hydrogen sulfide production in ship ballast and sewage tanks., (1998). https://apps.dtic.mil/sti/citations/ADA360622. Accessed 5 Apr 2023
Engineering ToolBox, Thermophysical Properties of Hydrogen sulfide, (2018). https://www.engineeringtoolbox.com/hydrogen-sulfide-H2S-properties-d_2034.html. Accessed 5 Apr 2023
J. Orlikowski, A. Jazdzewska, I. Uygur, R. Gospos, T. Olczak, K. Darowicki, Effect of wet hydrogen sulfide on carbon steels degradation in refinery based on case study. Arab. J. Sci. Eng. (2022). https://doi.org/10.1007/s13369-022-07154-0
W.C. Browning, H.F. Young, Process for Scavenging Hydrogen Sulfide in Aqueous Drilling Fluids and Method of Preventing Metallic Corrosion of Subterranean Well Drilling Apparatuses. U.S. Patent 3,928,211 A, (1975).
OSHA, Hydrogen Sulfide Hazards, Occupational Safety and Health Administration. (2005). https://www.osha.gov/hydrogen-sulfide/hazards. Accessed 27 Mar 2023
J.D. Ray, B.V. Randall, J.C. Parker, Use of reactive iron oxide to remove H2S from drilling fluid. J. Petrol. Technol. 31, 797–801 (1979). https://doi.org/10.2118/7498-PA
M.K. Amosa, I. A. Mohammed, S.A. Yaro, Sulphide Scavengers in Oil and Gas Industry: A Review. Nafta, 61, 85− 92 (2010).
A. Ahmed, S.A. Onaizi, S. Elkatatny, Improvement of hydrogen sulfide scavenging via the addition of monoethanolamine to water-based drilling fluids. ACS Omega 7, 28361–28368 (2022). https://doi.org/10.1021/acsomega.2c02890
S. Kafashi, M. Rasaei, G. Karimi, Effects of sugarcane and polyanionic cellulose on rheological properties of drilling mud: An experimental approach. Egypt. J. Pet. 26, 371–374 (2017). https://doi.org/10.1016/j.ejpe.2016.05.009
R. Saboori, S. Sabbaghi, A. Kalantariasl, D. Mowla, Improvement in filtration properties of water-based drilling fluid by nanocarboxymethyl cellulose/polystyrene core–shell nanocomposite. J. Pet. Explor. Prod. Technol. 8, 445–454 (2018). https://doi.org/10.1007/s13202-018-0432-9
A. Jinasena, R. Sharma, Estimation of mud losses during the removal of drill cuttings in oil drilling. SPE J. 25, 2162–2177 (2020). https://doi.org/10.2118/201230-PA
J. Yuan, Q. Li, J. Shen, K. Huang, G. Liu, J. Zhao, J. Duan, W. Jin, Hydrophobic-functionalized ZIF-8 nanoparticles incorporated PDMS membranes for high-selective separation of propane/nitrogen. Asia-Pac. J. Chem. Eng. 12, 110–120 (2017). https://doi.org/10.1002/APJ.2058
M. Bergaoui, M. Khalfaoui, A. Awadallah-F, S. Al-Muhtaseb, A review of the features and applications of ZIF-8 and its derivatives for separating CO2 and isomers of C3- and C4- hydrocarbons. J. Nat. Gas Sci. Eng. 96, (2021). https://doi.org/10.1016/J.JNGSE.2021.104289
Q. Song, S.K. Nataraj, M.V. Roussenova, J.C. Tan, D.J. Hughes, W. Li, P. Bourgoin, M.A. Alam, A.K. Cheetham, S.A. Al-Muhtaseb, E. Sivaniah, Zeolitic imidazolate framework (ZIF-8) based polymer nanocomposite membranes for gas separation. Energy Environ. Sci. 5, 8359–8369 (2012). https://doi.org/10.1039/C2EE21996D
C. Duan, Y. Yu, H. Hu, Recent progress on synthesis of ZIF-67-based materials and their application to heterogeneous catalysis. Green Energy Environ. 7, 3–15 (2022). https://doi.org/10.1016/J.GEE.2020.12.023
A. Samadi-Maybodi, S. Ghasemi, H. Ghaffari-Rad, A novel sensor based on Ag-loaded zeolitic imidazolate framework-8 nanocrystals for efficient electrocatalytic oxidation and trace level detection of hydrazine. Sens. Actuators B Chem. 220, 627–633 (2015). https://doi.org/10.1016/J.SNB.2015.05.127
A. Matsuoka, H. Matsumura, M. Odaka, N. Ogawa, T. Tanno, The transitional transmittance response of ZIF-8 gas adsorption observed using terahertz waves. E-J. Surf. Sci. Nanotech. 16, 142–144 (2018). https://doi.org/10.1380/EJSSNT.2018.142
E.E. Sann, Y. Pan, Z. Gao, S. Zhan, F. Xia, Highly hydrophobic ZIF-8 particles and application for oil-water separation. Sep. Purif. Technol. 206, 186–191 (2018). https://doi.org/10.1016/J.SEPPUR.2018.04.027
A. Katende, N.V. Boyou, I. Ismail, D.Z. Chung, F. Sagala, N. Hussein, M.S. Ismail, Improving the performance of oil based mud and water based mud in a high temperature hole using nanosilica nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 577, 645–673 (2019). https://doi.org/10.1016/J.COLSURFA.2019.05.088
M.I. Abduo, A.S. Dahab, H. Abuseda, A.M. AbdulAziz, M.S. Elhossieny, Comparative study of using water-based mud containing multiwall carbon nanotubes versus oil-based mud in HPHT fields. Egypt. J. Pet. 25, 459–464 (2016). https://doi.org/10.1016/J.EJPE.2015.10.008
P.K. Jha, V. Mahto, V.K. Saxena, Study the Rheological and Filtration Properties of Oil-in-Water Emulsion for its Application in Oil and Gas Well Drilling. J. Pet. Sci. Eng. 3, 25–30 (2013). https://www.stmjournals.com/index.php?journal=JoPET&page=article&op=view&path%5B%5D=4107. Accessed 8 July 2023.
A.A. Sulaimon, B.J. Adeyemi, M. Rahimi, Performance enhancement of selected vegetable oil as base fluid for drilling HPHT formation. J. Pet. Sci. Eng. 152, 49–59 (2017). https://doi.org/10.1016/j.petrol.2017.02.006
O.E. Agwu, A.N. Okon, F.D. Udoh, A comparative study of diesel oil and soybean oil as oil-based drilling mud. J. Pet. Eng. 2015, 1–10 (2015). https://doi.org/10.1155/2015/828451
Y. Li, K. Zhou, M. He, J. Yao, Synthesis of ZIF-8 and ZIF-67 using mixed-base and their dye adsorption. Microporous Mesoporous Mater. 234, 287–292 (2016). https://doi.org/10.1016/j.micromeso.2016.07.039
M. He, J. Yao, Q. Liu, K. Wang, F. Chen, H. Wang, Facile synthesis of zeolitic imidazolate framework-8 from a concentrated aqueous solution. Microporous Mesoporous Mater. 184, 55–60 (2014). https://doi.org/10.1016/j.micromeso.2013.10.003
R. Banerjee, A. Phan, B. Wang, C. Knobler, H. Furukawa, M. O’Keeffe, O.M. Yaghi, High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 319(2008), 939–943 (1979). https://doi.org/10.1126/SCIENCE.1152516/SUPPL_FILE/BANERJEE.SOM.PDF
Y. Zhang, Y. Jia, L. Hou, Synthesis of zeolitic imidazolate framework-8 on polyester fiber for PM2.5 removal. RSC Adv. 8, 31471–31477 (2018). https://doi.org/10.1039/c8ra06414h
H. Kaur, G.C. Mohanta, V. Gupta, D. Kukkar, S. Tyagi, Synthesis and characterization of ZIF-8 nanoparticles for controlled release of 6-mercaptopurine drug. J. Drug Deliv. Sci. Technol. 41, 106–112 (2017). https://doi.org/10.1016/j.jddst.2017.07.004
M. Davoodi, F. Davar, M.R. Rezayat, M.T. Jafari, M. Bazarganipour, A.E. Shalan, Synthesis and characterization of a new ZIF-67@MgAl2O4nanocomposite and its adsorption behaviour. RSC Adv. 11, 13245–13255 (2021). https://doi.org/10.1039/d1ra01056e
K.S. Park, Z. Ni, A.P. Côté, J.Y. Choi, R. Huang, F.J. Uribe-Romo, H.K. Chae, M. O’Keeffe, O.M. Yaghi, Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. U. S. A. 103, 10186–10191 (2006). https://doi.org/10.1073/PNAS.0602439103/SUPPL_FILE/02439SUPPAPPENDIX.PDF
Y. Pan, Y. Liu, G. Zeng, L. Zhao, Z. Lai, Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system. Chem. Commun. 47, 2071–2073 (2011). https://doi.org/10.1039/c0cc05002d
A. Schejn, L. Balan, V. Falk, L. Aranda, G. Medjahdi, R. Schneider, Controlling ZIF-8 nano- and microcrystal formation and reactivity through zinc salt variations. CrystEngComm 16, 4493–4500 (2014). https://doi.org/10.1039/c3ce42485e
L.S. Lai, Y.F. Yeong, N.C. Ani, K.K. Lau, A.M. Shariff, Effect of synthesis parameters on the formation of zeolitic imidazolate framework 8 (ZIF-8) nanoparticles for CO2 adsorption. Part. Sci. Technol. 32, 520–528 (2014). https://doi.org/10.1080/02726351.2014.920445
W. Cao, M. Han, L. Qin, Q. Jiang, J. Xu, Z. Lu, Y. Wang, Synthesis of zeolitic imidazolate framework-67 nanocube wrapped by graphene oxide and its application for supercapacitors. J. Solid State Electrochem. 23, 325–334 (2019). https://doi.org/10.1007/s10008-018-4138-1
W. Sun, X. Zhai, L. Zhao, Synthesis of ZIF-8 and ZIF-67 nanocrystals with well-controllable size distribution through reverse microemulsions. Chem. Eng. J. 289, 59–64 (2016). https://doi.org/10.1016/J.CEJ.2015.12.076
A.M. Alkadhem, M.A.A. Elgzoly, A. Alshami, S.A. Onaizi, Kinetics of CO2 capture by novel amine-functionalized magnesium oxide adsorbents. Colloids Surf A Physicochem. Eng. Asp. 616, 126258 (2021). https://doi.org/10.1016/j.colsurfa.2021.126258
M.K. Al-Sakkaf, S.A. Onaizi, Rheology, characteristics, stability, and pH-responsiveness of biosurfactant-stabilized crude oil/water nanoemulsions. Fuel 307, 121845 (2022). https://doi.org/10.1016/J.FUEL.2021.121845
S.A. Lateef, O.O. Ajumobi, S.A. Onaizi, Enzymatic desulfurization of crude oil and its fractions: a mini review on the recent progresses and challenges. Arab. J. Sci. Eng. 44, 5181–5193 (2019). https://doi.org/10.1007/S13369-019-03800-2/METRICS
S.A. Onaizi, Demulsification of crude oil/water nanoemulsions stabilized by rhamnolipid biosurfactant using enzymes and pH-swing. Sep. Purif. Technol. 259, 118060 (2021). https://doi.org/10.1016/J.SEPPUR.2020.118060
S.A. Onaizi, L. He, A.P.J. Middelberg, The construction, fouling and enzymatic cleaning of a textile dye surface. J. Colloid Interface Sci. 351, 203–209 (2010). https://doi.org/10.1016/J.JCIS.2010.07.030
S.A. Onaizi, A.S. Malcolm, L. He, A.P.J. Middelberg, Directed disassembly of an interfacial rubisco protein network. Langmuir 23, 6336–6341 (2007). https://doi.org/10.1021/LA700378Q/ASSET/IMAGES/LARGE/LA700378QF00007.JPEG
S.A. Onaizi, L. He, A.P.J. Middelberg, Proteolytic cleaning of a surface-bound rubisco protein stain. Chem. Eng. Sci. 64, 3868–3878 (2009). https://doi.org/10.1016/J.CES.2009.05.027
L. He, A.S. Malcolm, M. Dimitrijev, S.A. Onaizi, H.H. Shen, S.A. Holt, A.F. Dexter, R.K. Thomas, A.P.J. Middelberg, Cooperative tuneable interactions between a designed peptide biosurfactant and positional isomers of SDOBS at the air - Water interface. Langmuir 25, 4021–4026 (2009). https://doi.org/10.1021/LA802825C/ASSET/IMAGES/LARGE/LA-2008-02825C_0007.JPEG
S.A. Onaizi, M.S. Nasser, N.M.A. Al-Lagtah, Self-assembly of a surfactin nanolayer at solid–liquid and air–liquid interfaces. Eur. Biophys. J. 45, 331–339 (2016). https://doi.org/10.1007/S00249-015-1099-5/FIGURES/5
S.A. Onaizi, M.S. Nasser, N.M.A. Al-Lagtah, Benchmarking the Self-assembly of surfactin biosurfactant at the liquid-air interface to those of synthetic surfactants. J. Surfactants Deterg. 19, 645–652 (2016). https://doi.org/10.1007/S11743-016-1796-9/FIGURES/3
S.A. Onaizi, Dynamic surface tension and adsorption mechanism of surfactin biosurfactant at the air–water interface. Eur. Biophys. J. 47, 631–640 (2018). https://doi.org/10.1007/S00249-018-1289-Z/FIGURES/8
S.A. Onaizi, M. Alsulaimani, M.K. Al-Sakkaf, S.A. Bahadi, M. Mahmoud, A. Alshami, Crude oil/water nanoemulsions stabilized by biosurfactant: Stability and pH-Switchability. J. Pet. Sci. Eng. 198, 108173 (2021). https://doi.org/10.1016/J.PETROL.2020.108173
A.M. Alkadhem, M.A.A. Elgzoly, S.A. Onaizi, Novel amine-functionalized magnesium oxide adsorbents for CO2 capture at ambient conditions. J. Environ. Chem. Eng. 8, 103968 (2020). https://doi.org/10.1016/j.jece.2020.103968
S.A. Onaizi, Statistical analyses of the effect of rhamnolipid biosurfactant addition on the enzymatic removal of Bisphenol A from wastewater. Biocatal. Agric. Biotechnol. 32, 101929 (2021). https://doi.org/10.1016/J.BCAB.2021.101929
M. Alshabib, S.A. Onaizi, Effects of surface active additives on the enzymatic treatment of phenol and its derivatives: a mini review. Curr. Pollut. Rep. 5, 52–65 (2019). https://doi.org/10.1007/S40726-019-00105-8/TABLES/3
M. Alshabib, S.A. Onaizi, Enzymatic remediation of bisphenol a from wastewaters: effects of biosurfactant, anionic, cationic, nonionic, and polymeric additives. Water Air Soil Pollut. 231, 1–13 (2020). https://doi.org/10.1007/S11270-020-04806-5/TABLES/1
A. Hezam, Q.A. Drmosh, D. Ponnamma, M.A. Bajiri, M. Qamar, K. Namratha, M. Zare, M.B. Nayan, S.A. Onaizi, K. Byrappa, Strategies to enhance ZnO photocatalyst’s performance for water treatment: a comprehensive review. Chem. Record. 22, e202100299 (2022). https://doi.org/10.1002/TCR.202100299
H.S. Almarouf, M.S. Nasser, M.J. Al-Marri, M. Khraisheh, S.A. Onaizi, Demulsification of stable emulsions from produced water using a phase separator with inclined parallel arc coalescing plates. J. Pet. Sci. Eng. 135, 16–21 (2015). https://doi.org/10.1016/J.PETROL.2015.08.005
S.A. Onaizi, M. Alshabib, The degradation of bisphenol A by laccase: Effect of biosurfactant addition on the reaction kinetics under various conditions. Sep. Purif. Technol. 257, 117785 (2021). https://doi.org/10.1016/J.SEPPUR.2020.117785
E.E. Okoro, A.A. Zuokumor, I.S. Okafor, K.C. Igwilo, K.B. Orodu, Determining the optimum concentration of multiwalled carbon nanotubes as filtrate loss additive in field-applicable mud systems. J. Pet. Explor. Prod. Technol. 10, 429–438 (2020). https://doi.org/10.1007/S13202-019-0740-8/FIGURES/9
S.A. Onaizi, M.A. Gawish, M. Murtaza, I. Gomaa, Z. Tariq, M. Mahmoud, H 2 S scavenging capacity and rheological properties of water-based drilling muds. ACS Omega 5, 30729–30739 (2020). https://doi.org/10.1021/acsomega.0c04953
A. Mirzaei-Paiaman, M. Masihi, A.M. Paiaman, M.K.G. Al-Askari, B. Salmani, B.D. Al-Anazi, M. Masihi, Effect of Drilling Fluid Properties on Rate of Penetration, (2009). https://www.researchgate.net/publication/267959339
N.V. Boyou, I. Ismail, W.R. Wan Sulaiman, A. Sharifi Haddad, N. Husein, H.T. Hui, K. Nadaraja, Experimental investigation of hole cleaning in directional drilling by using nano-enhanced water-based drilling fluids. J. Pet. Sci. Eng. 176, 220–231 (2019). https://doi.org/10.1016/J.PETROL.2019.01.063
A.H. Salih, H. Bilgesu, Investigation of Rheological and Filtration Properties of Water-Based Drilling Fluids Using Various Anionic Nanoparticles, SPE Western Regional Meeting Proceedings. 2017-April (2017) 837–857. https://doi.org/10.2118/185638-MS
A. Bardhan, R. Gandhi, H. Kesarwani, S. Vats, S. Sharma, S. Kumar, Performance evaluation of novel silane coated nanoparticles as an additive for high-performance drilling fluid applications, (2023). https://doi.org/10.2523/IPTC-22878-MS
S.R. Smith, R. Rafati, A. Sharifi Haddad, A. Cooper, H. Hamidi, Application of aluminium oxide nanoparticles to enhance rheological and filtration properties of water based muds at HPHT conditions. Colloids Surf A Physicochem. Eng. Asp. 537, 361–371 (2018). https://doi.org/10.1016/J.COLSURFA.2017.10.050
Acknowledgements
This work was supported by the Deanship of Research Oversight and Coordination (DROC) at King Fahd University of Petroleum and Minerals (KFUPM) in the terms of Research Grant #DF191027.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iddrisu, M., Bahadi, S.A., Al-Sakkaf, M.K. et al. Harnessing zeolitic imidazolate framework-8 (ZIF-8) nanoparticles for enhancing H2S scavenging capacity of waste vegetable oil-based drilling fluids. emergent mater. 7, 591–602 (2024). https://doi.org/10.1007/s42247-023-00535-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-023-00535-7